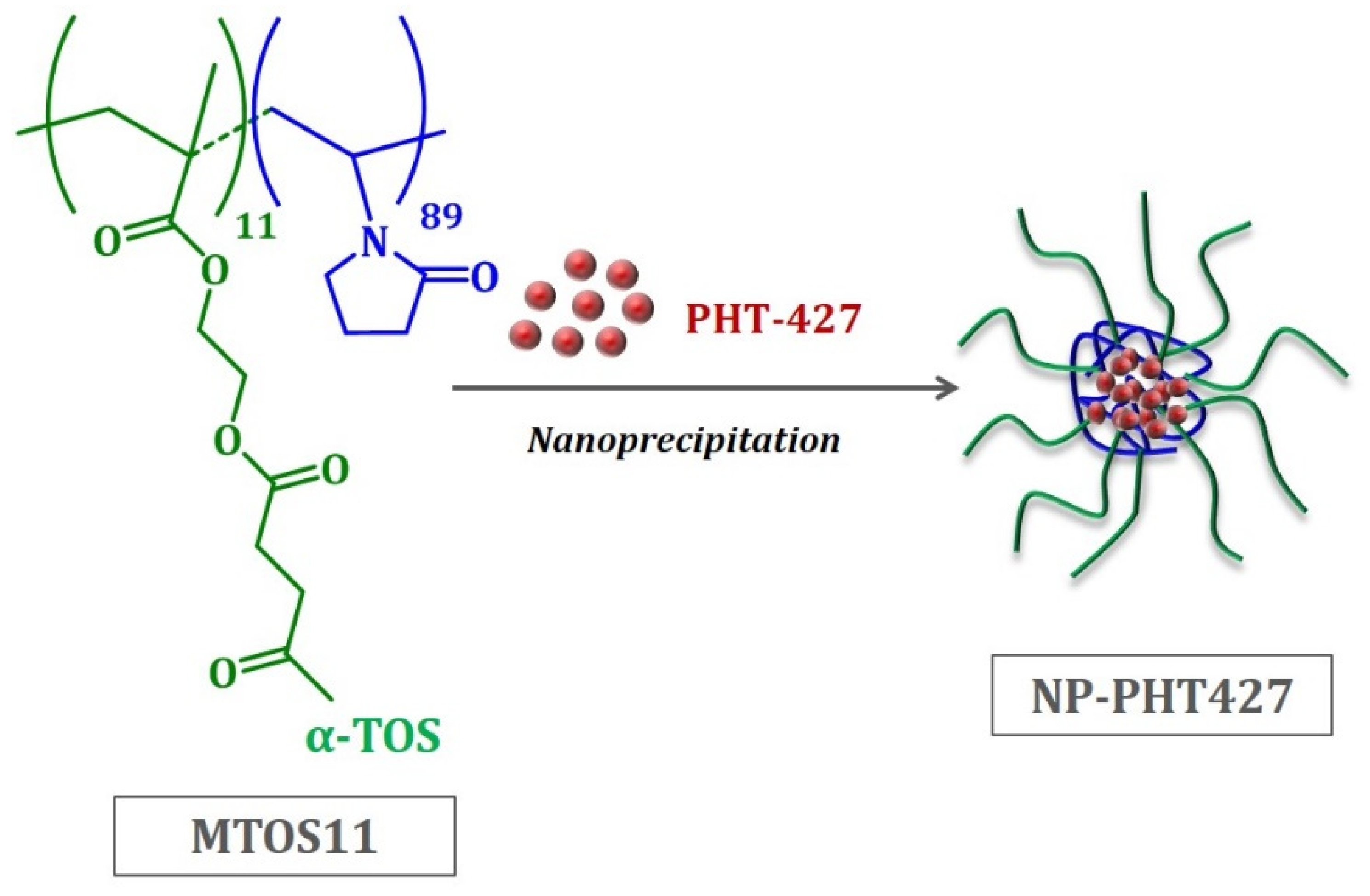
Currently, new treatments are required to supplement the current standard of care for head and neck squamous cell carcinoma (HNSCC). The phosphatidylinositol3-kinase (PI3K) signaling pathway is commonly altered and activated in HNSCC. PHT-427 is a dual PI3K-mammalian target of the AKT/PDK1 inhibitor; however, to the best of our knowledge, the effect of the PHT-427 inhibitor on HNSCC has not been investigated. This study aims to evaluate the antitumoral effect of PHT-427-loaded polymeric nanoparticles based on α-tocopheryl succinate (α-TOS). The in vitro activity of PHT-427 was tested in hypopharynx carcinoma squamous cells (FaDu) to measure the cell viability, PI3KCA/AKT/PDK1 gene expression, and PI3KCA/AKT/PDK1 levels. Apoptosis, epidermal growth factor receptor (EGFR), and reactive oxygen species (ROS) were also measured. The presence of PHT-427 significantly enhances its antiproliferative and proapoptotic activity by inactivating the PI3K/AKT/PDK1 pathway. Nanoparticles (NPs) effectively suppress AKT/PDK1 expression. Additionally, NPs loaded with PHT-427 produce high oxidative stress levels that induce apoptosis. In conclusion, these results are promising in the use of this nanoformulation as a PHT-427 delivery system for effective HNSCC treatment.
Paclitaxel-loaded polymeric nanoparticles based on α-tocopheryl succinate for the treatment of head and neck squamous cell carcinoma: in vivo murine model
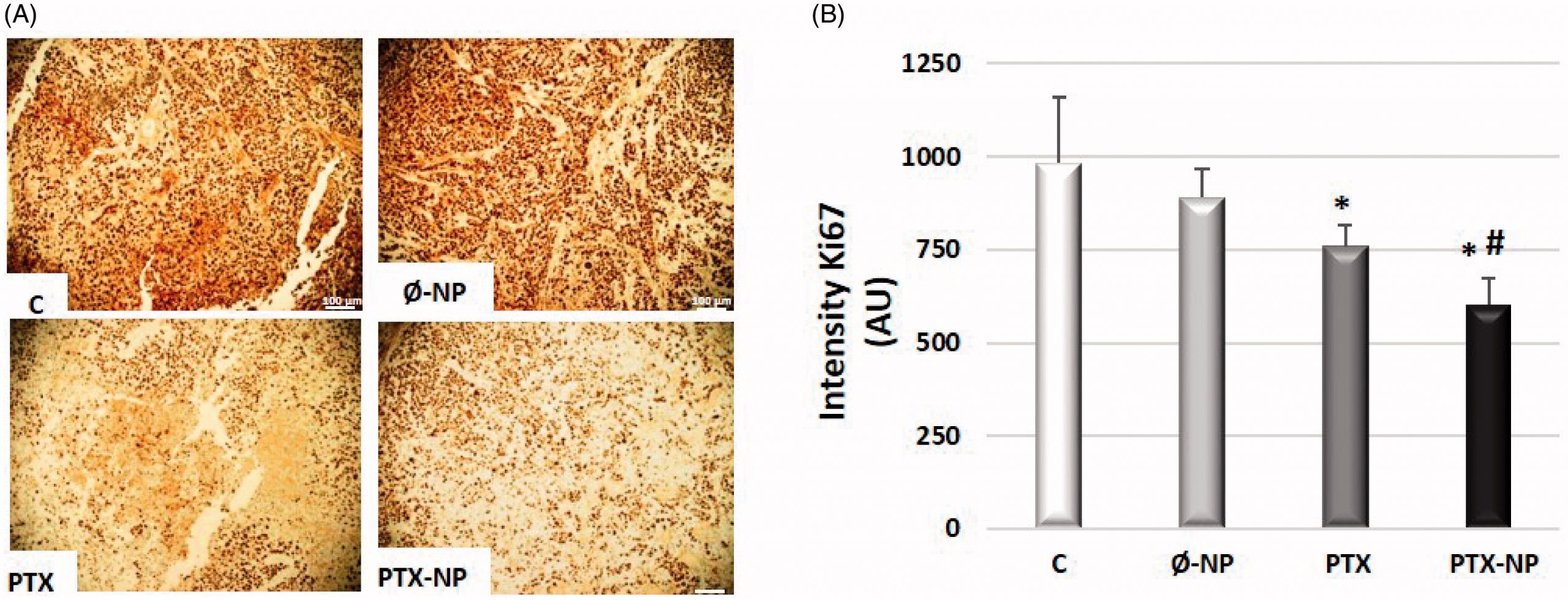
The prognosis of patients with recurrent or metastatic head and neck squamous cell cancer (HNSCC) is generally poor. New treatments are required to supplement the current standard of care. Paclitaxel (PTX), an effective chemotherapeutic for HNSCC, has serious side effects. A polymeric nanocarrier system was developed for the delivery of PTX to improve HNSCC treatment. This study aimed to evaluate the antitumor efficacy of PTX-loaded polymeric nanoparticles based on α-TOS (PTX-NPs) administered by direct intratumoral injection into a Hypopharynx carcinoma squamous cells (FaDu) tumor xenograft mouse model. The nanocarrier system based on block copolymers of polyethylene glycol (PEG) and a methacrylic derivative of α-TOS was synthesized and PTX was loaded into the delivery system. Tumor volume was measured to evaluate the antitumor effect of the PTX-NPs. The relative mechanisms of apoptosis, cell proliferation, growth, angiogenesis, and oxidative and nitrosative stress were detected by Western blotting, fluorescent probes, and immunohistochemical analysis. The antitumor activity results showed that compared to free PTX, PTX-NPs exhibited much higher antitumor efficacy and apoptosis-inducing in a FaDu mouse xenograft model and demonstrated an improved safety profile. Ki-67, EGFR, and angiogenesis markers (Factor VIII, CD31, and CD34) expression were significantly lower in the PTX-NPs group compared with other groups (p < .05). Also, PTX-NPs induced oxidative and nitrosative stress in tumor tissue. Direct administration of PTX-loaded polymeric nanoparticles based on α-Tocopheryl Succinate at the tumor sites, proved to be promising for HNSCC therapy.
α-Tocopheryl Succinate-Based Polymeric Nanoparticles for the Treatment of Head and Neck Squamous Cell Carcinoma
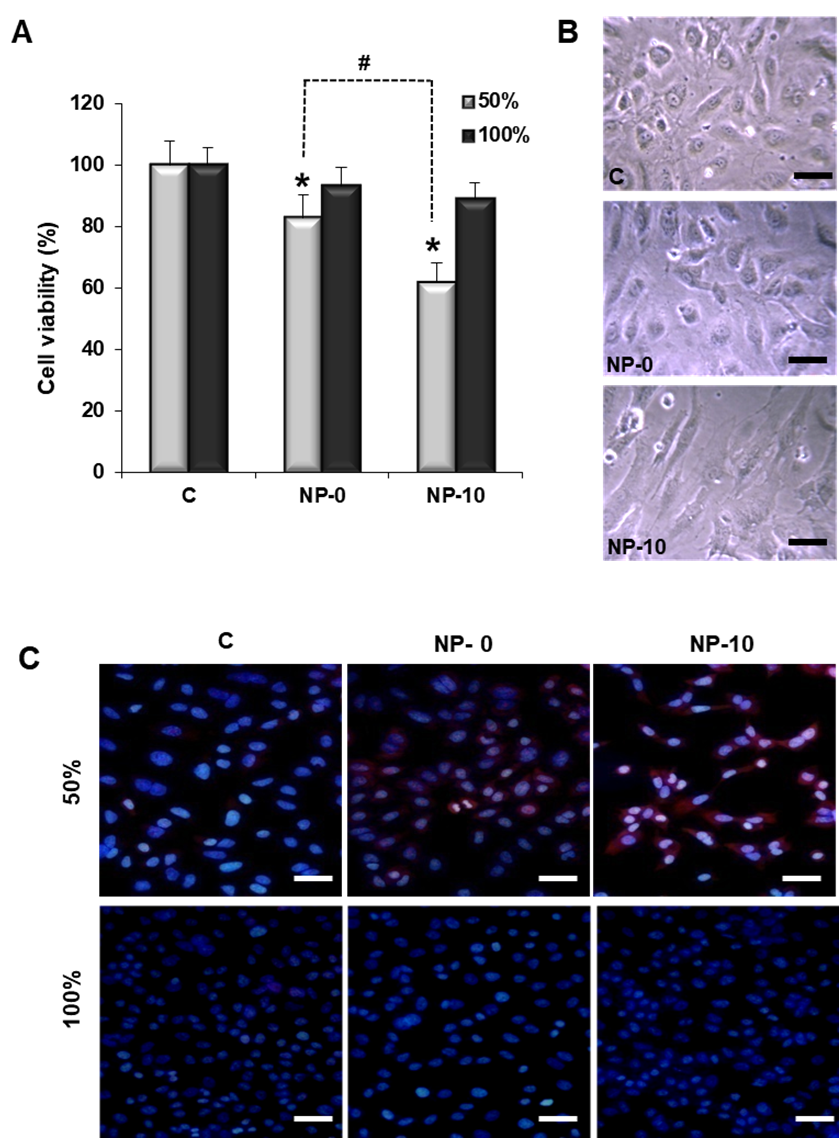
The aim of this work is to study, in an in vitro head and neck squamous cell carcinomas model the anti-angiogenic and anti-migratory properties of self-assembled polymeric nanoparticles (NPs) with demonstrated selective anticancer activity. The NPs are based on α-tocopheryl succinate (α-TOS) encapsulated in the hydrophobic core of the NPs. We analyzed the effect of the newly synthetized α-TOS-loaded NPs in proliferating endothelial cells and hypopharynx carcinoma squamous cells and measured markers of angiogenesis, apoptosis and reactive oxygen species (ROS). α-TOS-loaded NPs suppressed angiogenesis by inducing accumulation of ROS and inducing apoptosis of proliferating endothelial cells. These NPs also decrease the number and quality of capillary-like tubes in an in vitro three-dimensional (3D) experiment, decrease the production of the pro-angiogenic vascular endothelial growth factor and down-regulate the expression of its receptor. The anti-migratory efficacy of α-TOS is corroborated in hypopharynx carcinoma cells by decreasing the secretion of matrix metalloproteases 2 and 9 (MMP-2 and MMP-9) and inhibiting cell migration. These results confirm that α-TOS-based NPs not only present anticancer properties, but also antiangiogenic properties, therefore making them promising candidates for multi-active combinatorial anticancer therapy.