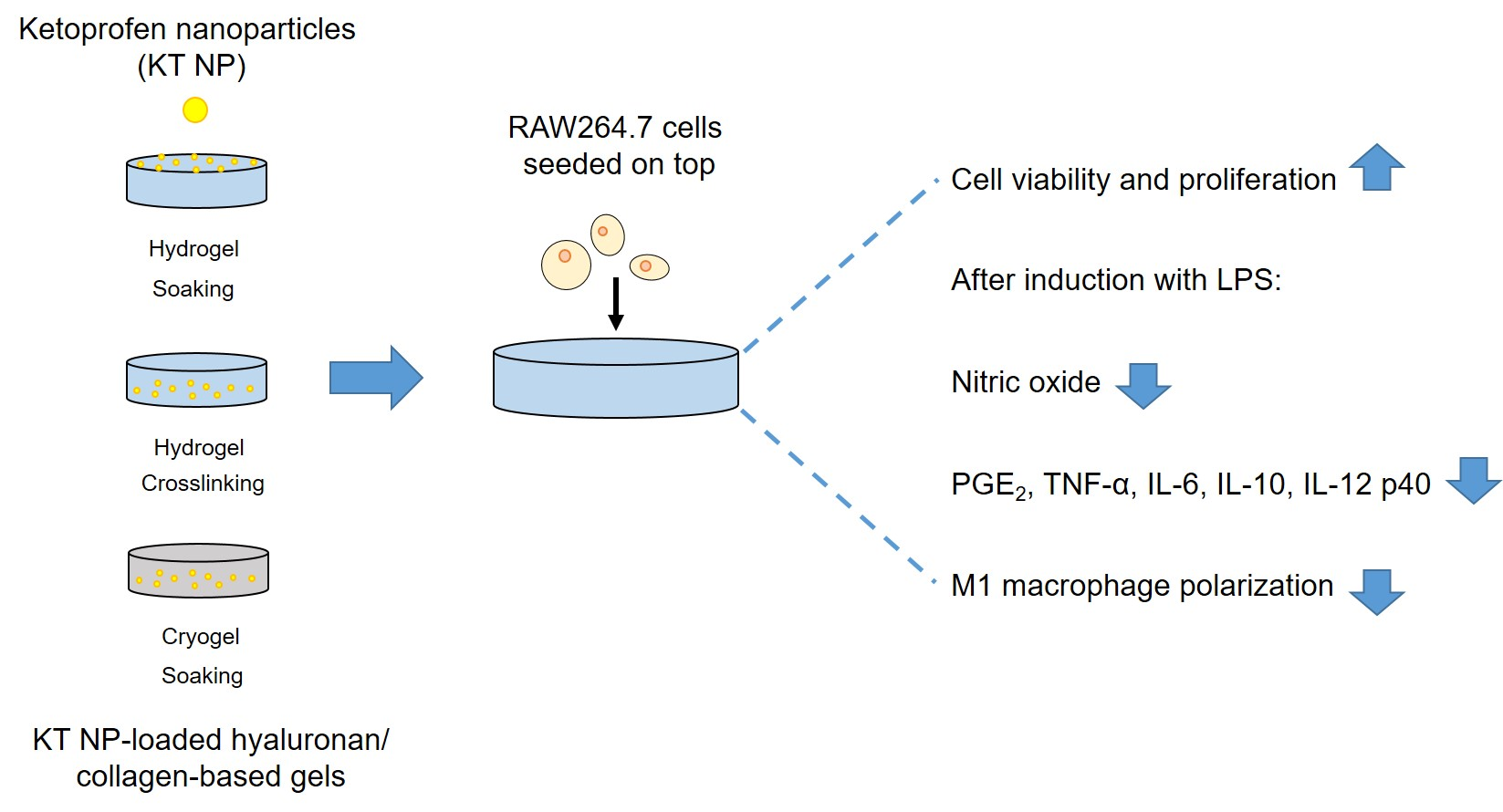
Current limitations of wound dressings for treating chronic wounds require the development of novel approaches. One of these is the immune-centered approach, which aims to restore the pro-regenerative and anti-inflammatory properties of macrophages. Under inflammatory conditions, ketoprofen nanoparticles (KT NP) can reduce pro-inflammatory markers of macrophages and increase anti-inflammatory cytokines. To assess their suitability as part of wound dressings, these NP were combined with hyaluronan (HA)/collagen-based hydro- (HG) and cryogels (CG). Different HA, NP concentrations and loading techniques for NP incorporation were used. The NP release, gel morphology and mechanical properties were studied. Generally, colonialization of the gels with macrophages resulted in high cell viability and proliferation. Furthermore, direct contact of the NP to the cells reduced the level of nitric oxide (NO). The formation of multinucleated cells on the gels was low and further decreased by the NP. For the HG that produced the highest reduction in NO, extended ELISA studies showed reduced levels of the pro-inflammatory markers PGE2, IL-12 p40, TNF-α, and IL-6. Thus, HA/collagen-based gels con-taining KT NP may represent a novel therapeutic approach for treating chronic wounds. Whether effects observed in vitro translate into a favorable profile on skin regeneration in vivo will require rigorous testing.
Chemically crosslinked hyaluronic acid-chitosan hydrogel for application on cartilage regeneration
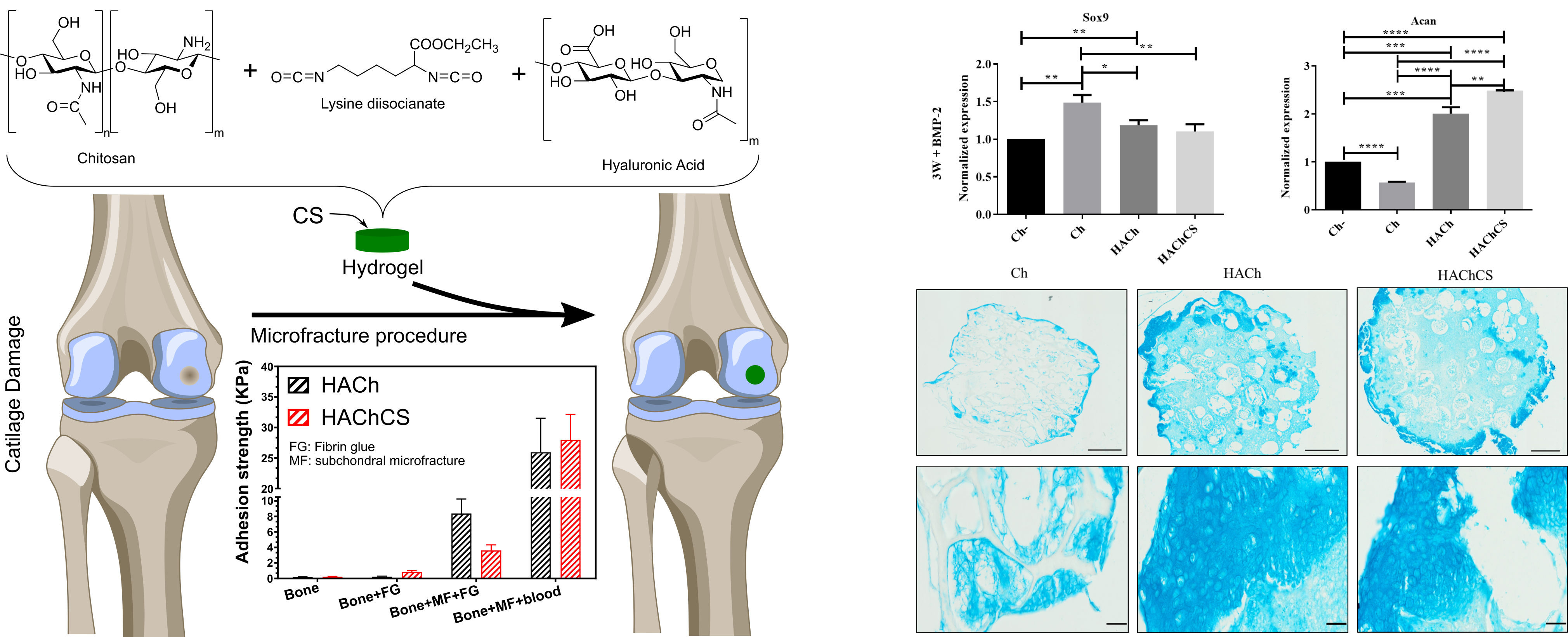
Articular cartilage is an avascular tissue that lines the ends of bones in diarthrodial joints, serves as support, acts as a shock absorber, and facilitates joint’s motion. It is formed by chondrocytes immersed in a dense extracellular matrix (principally composed of aggrecan linked to hyaluronic acid long chains). Damage to this tissue is usually associated with traumatic injuries or age-associated processes that often lead to discomfort, pain and disability in our aging society. Currently, there are few surgical alternatives to treat cartilage damage: the most commonly used is the microfracture procedure, but others include limited grafting or alternative chondrocyte implantation techniques, however, none of them completely restore a fully functional cartilage. Here we present the development of hydrogels based on hyaluronic acid and chitosan loaded with chondroitin sulfate by a new strategy of synthesis using biodegradable di-isocyanates to obtain an interpenetrated network of chitosan and hyaluronic acid for cartilage repair. These scaffolds act as delivery systems for the chondroitin sulfate and present mucoadhesive properties, which stabilizes the clot of microfracture procedures and promotes superficial chondrocyte differentiation favoring a true articular cellular colonization of the cartilage. This double feature potentially improves the microfracture technique and it will allow the development of next-generation therapies against articular cartilage damage
Biomimetic Gradient Scaffolds Containing Hyaluronic Acid and Sr/Zn Folates for Osteochondral Tissue Engineering
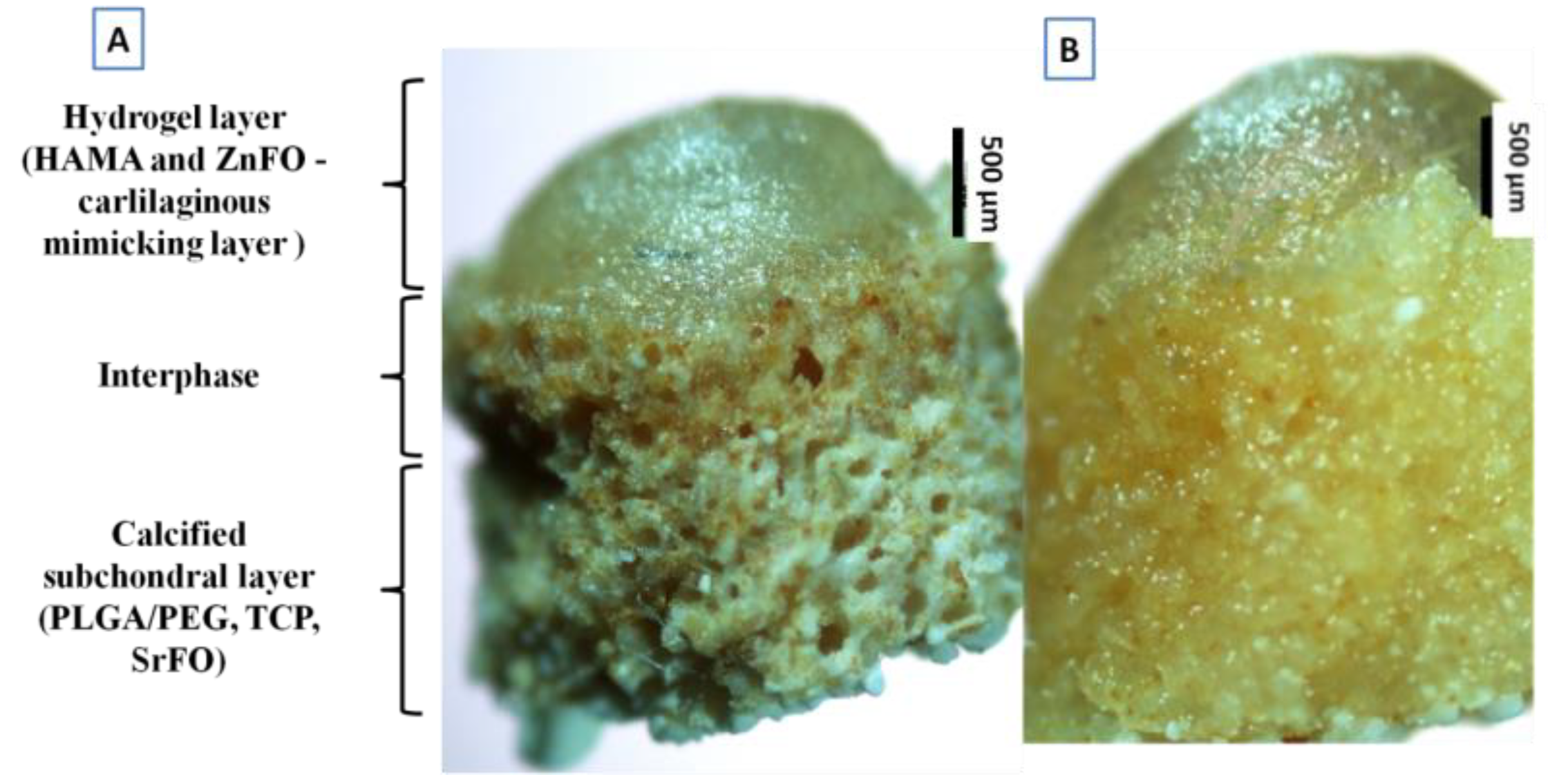
Regenerative therapies based on tissue engineering are becoming the most promising alternative for the treatment of osteoarthritis and rheumatoid arthritis. However, regeneration of full-thickness articular osteochondral defects that reproduces the complexity of native cartilage and osteochondral interface still remains challenging. Hence, in this work, we present the fabrication, physic-chemical characterization, and in vitro and in vivo evaluation of biomimetic hierarchical scaffolds that mimic both the spatial organization and composition of cartilage and the osteochondral interface. The scaffold is composed of a composite porous support obtained by cryopolymerization of poly(ethylene glycol) dimethacrylate (PEGDMA) in the presence of biodegradable poly(D,L-lactide-co-glycolide) (PLGA), bioactive tricalcium phosphate β-TCP and the bone promoting strontium folate (SrFO), with a gradient biomimetic photo-polymerized methacrylated hyaluronic acid (HAMA) based hydrogel containing the bioactive zinc folic acid derivative (ZnFO). Microscopical analysis of hierarchical scaffolds showed an open interconnected porous open microstructure and the in vitro behaviour results indicated high swelling capacity with a sustained degradation rate. In vitro release studies during 3 weeks indicated the sustained leaching of bioactive compounds, i.e., Sr2+, Zn2+ and folic acid, within a biologically active range without negative effects on human osteoblast cells (hOBs) and human articular cartilage cells (hACs) cultures. In vitro co-cultures of hOBs and hACs revealed guided cell colonization and proliferation according to the matrix microstructure and composition. In vivo rabbit-condyle experiments in a critical-sized defect model showed the ability of the biomimetic scaffold to promote the regeneration of cartilage-like tissue over the scaffold and neoformation of osteochondral tissue
Hyaluronic acid (HA)-coated naproxen-nanoparticles selectively target breast cancer stem cells through COX-independent pathways
Cytotoxic chemotherapy continues to be the main therapeutic option for patients with metastatic breast cancer. Several studies have reported a significant association between chronic inflammation, carcinogenesis and the presence of cancer stem cells (CSC). We hypothesized that the use of non-steroidal anti-inflammatory drugs targeted to the CSC population could help reducing tumor progression and dissemination in otherwise hard to treat metastatic breast cancer. Within this study cationic naproxen (NAP)-bearing polymeric nanoparticles (NPs) were obtained by self-assembly and they were coated with hyaluronic acid (HA) via electrostatic interaction. HA-coated and uncoated NAP-bearing NPs with different sizes were produced by changing the ionic strength of the aqueous preparation solutions (i.e. 300 and 350 nm or 100 and 130 nm in diameter, respectively). HA-NPs were fully characterized in terms of physicochemical parameters and biological response in cancer cells, macrophages and endothelial cells. Our results revealed that HA-coating of NPs provided a better control in NAP release and improved their hemocompatibility, while ensuring a strong CSC-targeting in MCF-7 breast cancer cells. Furthermore, the best polymeric NPs formulation significantly (p < 0.001) reduced MCF-7 cells viability when compared to free drug (i.e. 45 ± 6% for S-HA-NPs and 87 ± 10% for free NAP) by p53-dependent induction of apoptosis; and the migration of these cell line was also significantly (p < 0.01) reduced by the nano-formulated NAP (i.e. 76.4% of open wound for S-HA-NPs and 61.6% of open wound for NAP). This increased anti-cancer activity of HA-NAP-NPs might be related to the induction of apoptosis through alterations of the GSK-3β-related COX-independent pathway. Overall, these findings suggest that the HA-NAP-NPs have the potential to improve the treatment of advanced breast cancer by increasing the anti-proliferative effect of NAP within the CSC subpopulation.
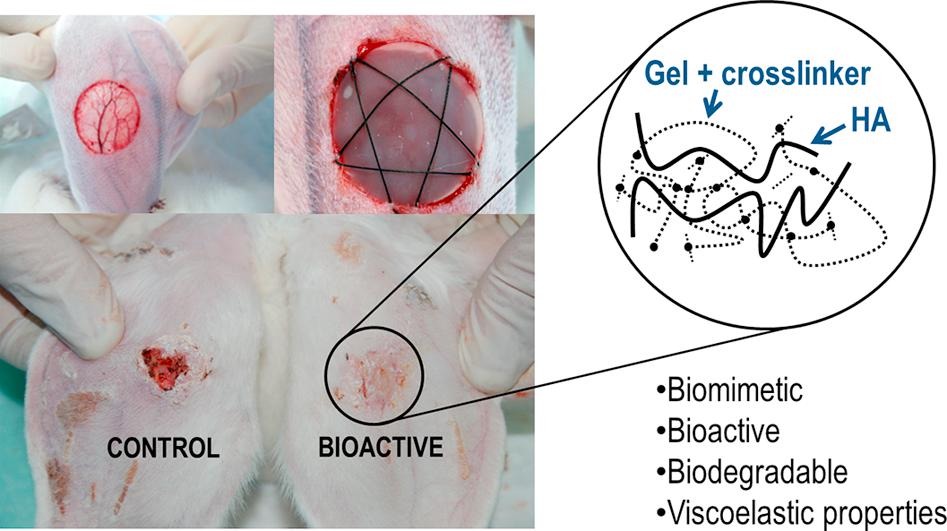
Contribution of bioactive hyaluronic acid and gelatin to regenerative medicine. Methodologies of gels preparation and advanced applications
The functionality and reactivity of polysaccharides, and in particular hyaluronic acid, in combination with proteins like gelatin, collagen and many others, offer very interesting opportunities for the new trends in regenerative medicine. In this review is described the relevance of gelatin (Gel) and hyaluronic acid (HA) biopolymers in the field of tissue engineering due to the excellent response of these biomimetic materials and their bioactive and biodegradable character in the human body. In addition, it is reported an overview of the most relevant crosslinking processes and agents that are being developed for regenerative medicine, including different hydrogel modifications as well as several interesting and advanced applications. The growing of clinical applications of these macromolecular components as assemblies opens new and advanced opportunities in regenerative medicine and drug delivery fields.
Free download until 16.12.2017: https://authors.elsevier.com/c/1VyP~3GBFCik9