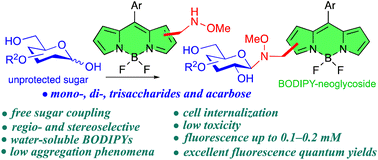
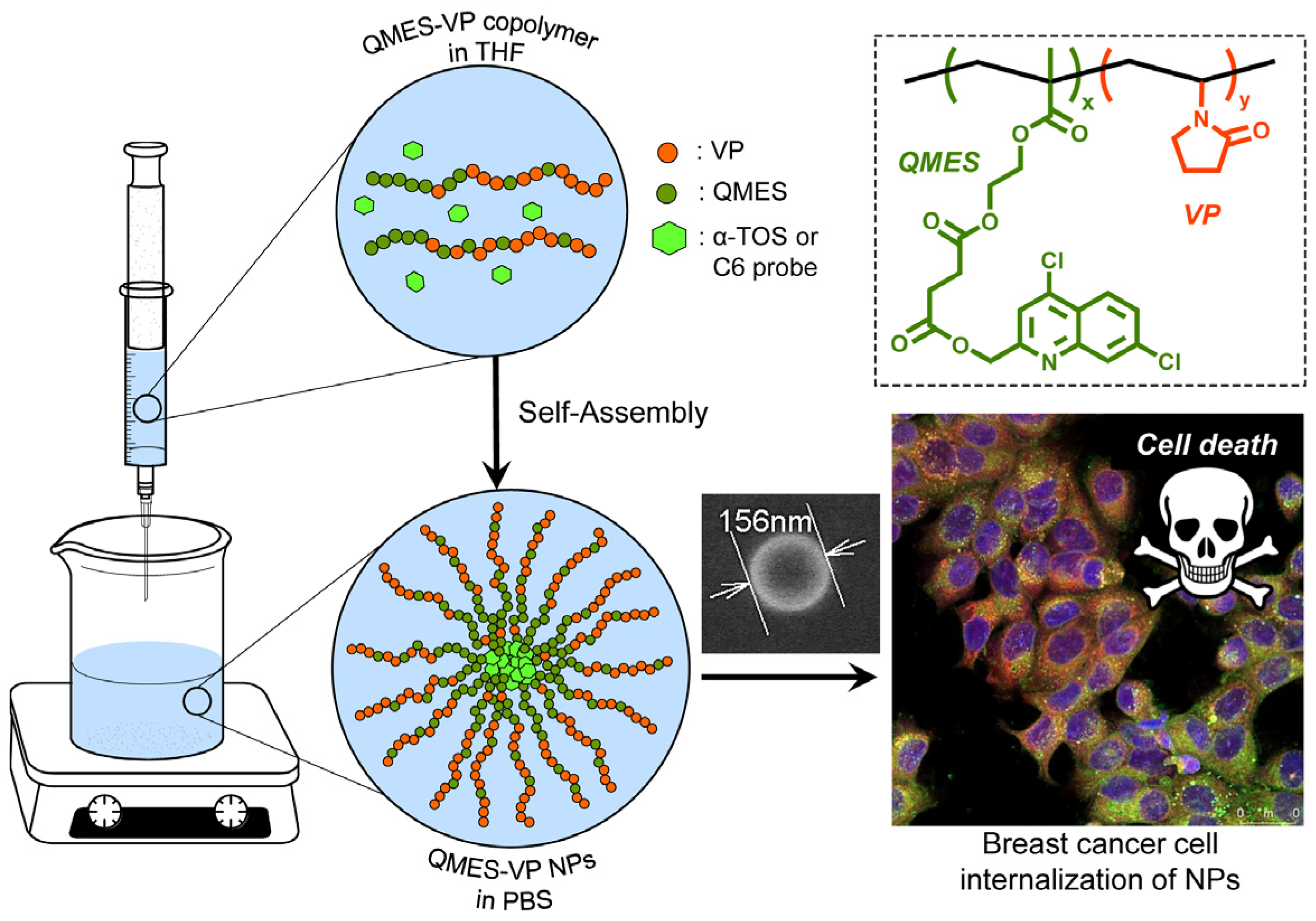
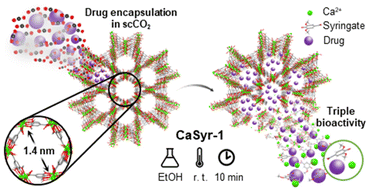
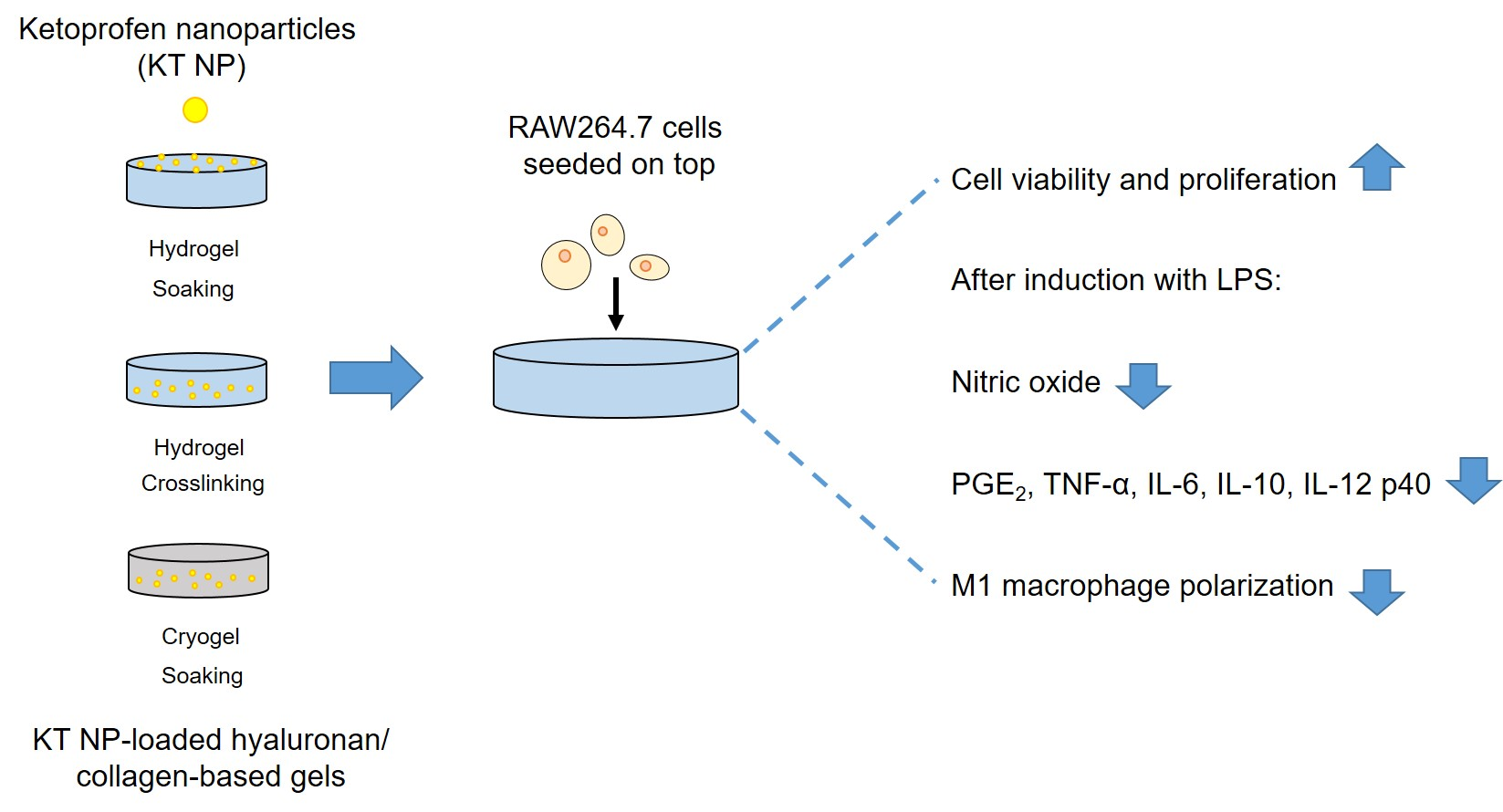
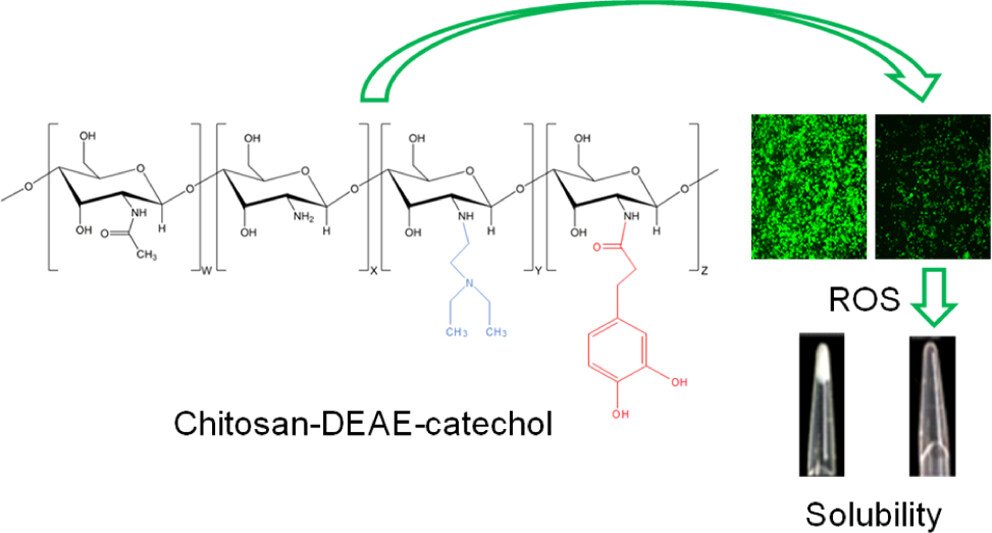
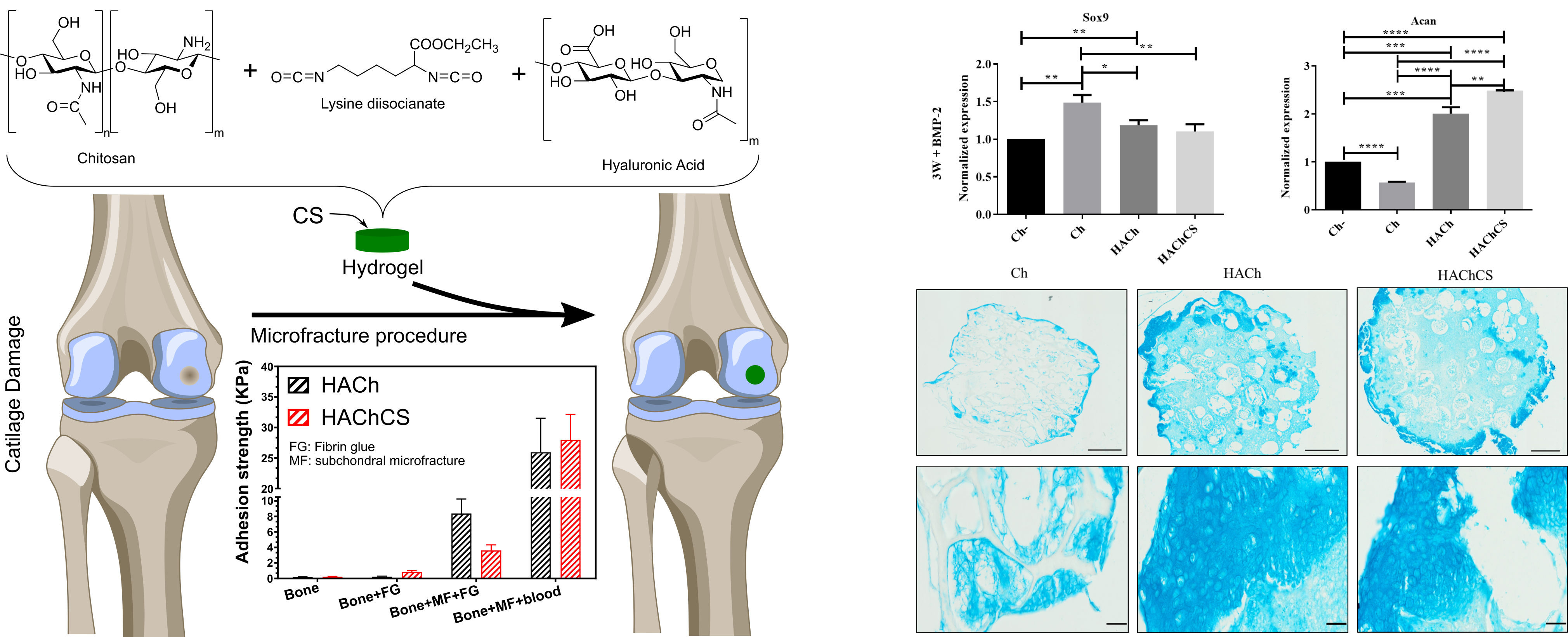
In order to improve the water solubility and, therefore, bioavailability and therapeutic activity of anticancer hydrophobic drug α-tocopherol succinate (α-TOS), in this work, copolymers were synthesized via free radicals from QMES (1-[4,7-dichloroquinolin-2-ylmethyl]-4-methacryloyloxyethyl succinate) and VP (N-vinyl-2-pirrolidone) using different molar ratios, and were used to nanoencapsulate and deliver α-TOS into cancer cells MCF-7. QMES monomer was chosen because the QMES pendant group in the polymer tends to hydrolyze to form free 4,7-dichloro-2-quinolinemethanol (QOH), which also, like α-TOS, exhibit anti-proliferative effects on cancerous cells. From the QMES-VP 30:70 (QMES-30) and 40:60 (QMES-40) copolymers obtained, it was possible to prepare aqueous suspensions of empty nanoparticles (NPs) loaded with α-TOS by nanoprecipitation. The diameter and encapsulation efficiency (%EE) of the QMES-30 NPs loaded with α-TOS were 128.6 nm and 52%; while for the QMES-40 NPs loaded with α-TOS, they were 148.8 nm and 65%. The results of the AlamarBlue assay at 72 h of treatment show that empty QMES-30 NPs (without α-TOS) produced a marked cytotoxic effect on MCF-7 breast cancer cells, corresponding to an IC50 value of 0.043 mg mL−1, and importantly, they did not exhibit cytotoxicity against healthy HUVEC cells. Furthermore, NP-QMES-40 loaded with α-TOS were cytotoxic with an IC50 value of 0.076 mg mL−1, demonstrating a progressive release of α-TOS; however, the latter nanoparticles were also cytotoxic to healthy cells in the range of the assayed concentrations. These results contribute to the search for a new polymeric nanocarrier of QOH, α-TOS or other hydrophobic drugs for the treatment of cancer or others diseases treatable with these drugs.