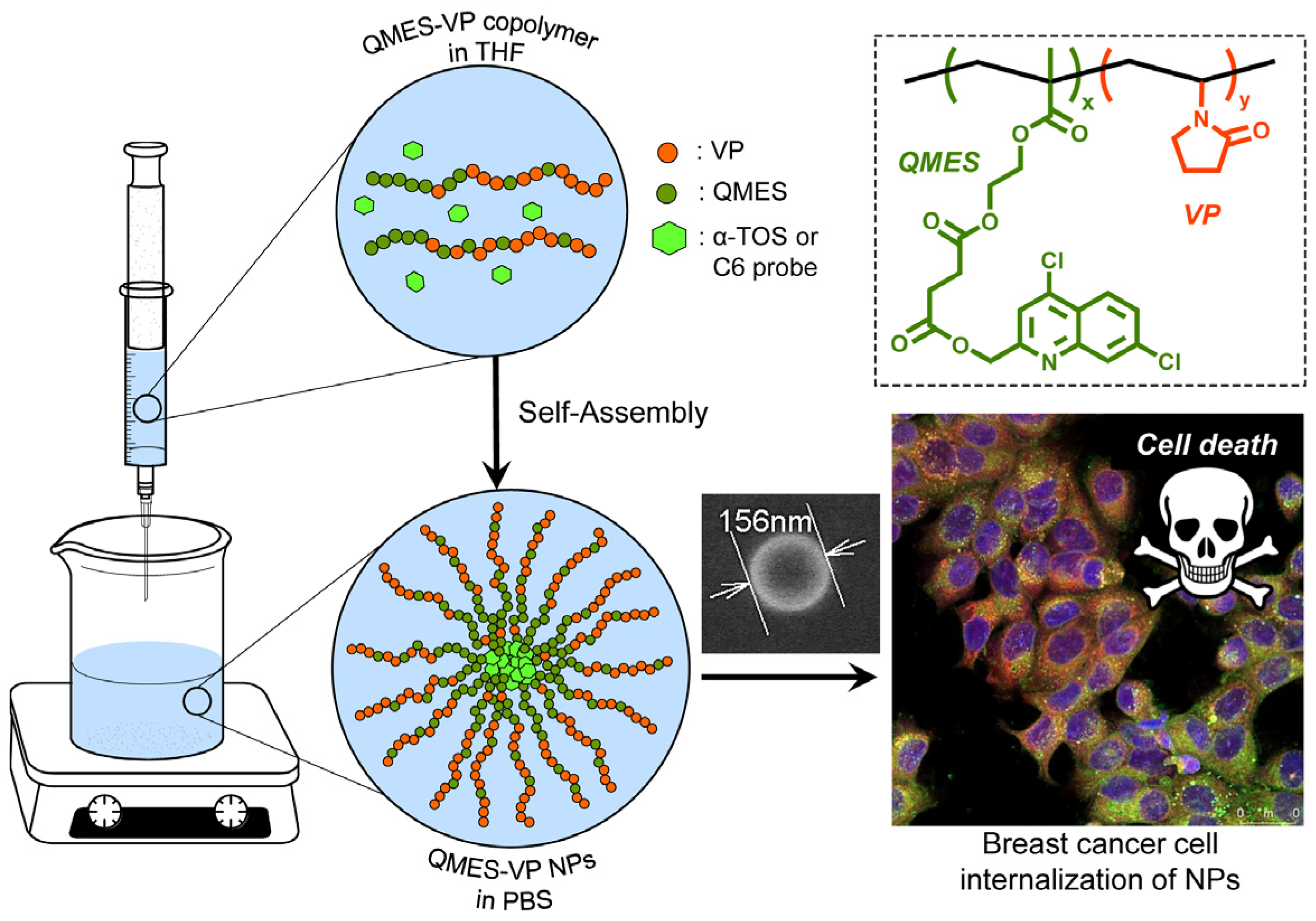
In order to improve the water solubility and, therefore, bioavailability and therapeutic activity of anticancer hydrophobic drug α-tocopherol succinate (α-TOS), in this work, copolymers were synthesized via free radicals from QMES (1-[4,7-dichloroquinolin-2-ylmethyl]-4-methacryloyloxyethyl succinate) and VP (N-vinyl-2-pirrolidone) using different molar ratios, and were used to nanoencapsulate and deliver α-TOS into cancer cells MCF-7. QMES monomer was chosen because the QMES pendant group in the polymer tends to hydrolyze to form free 4,7-dichloro-2-quinolinemethanol (QOH), which also, like α-TOS, exhibit anti-proliferative effects on cancerous cells. From the QMES-VP 30:70 (QMES-30) and 40:60 (QMES-40) copolymers obtained, it was possible to prepare aqueous suspensions of empty nanoparticles (NPs) loaded with α-TOS by nanoprecipitation. The diameter and encapsulation efficiency (%EE) of the QMES-30 NPs loaded with α-TOS were 128.6 nm and 52%; while for the QMES-40 NPs loaded with α-TOS, they were 148.8 nm and 65%. The results of the AlamarBlue assay at 72 h of treatment show that empty QMES-30 NPs (without α-TOS) produced a marked cytotoxic effect on MCF-7 breast cancer cells, corresponding to an IC50 value of 0.043 mg mL−1, and importantly, they did not exhibit cytotoxicity against healthy HUVEC cells. Furthermore, NP-QMES-40 loaded with α-TOS were cytotoxic with an IC50 value of 0.076 mg mL−1, demonstrating a progressive release of α-TOS; however, the latter nanoparticles were also cytotoxic to healthy cells in the range of the assayed concentrations. These results contribute to the search for a new polymeric nanocarrier of QOH, α-TOS or other hydrophobic drugs for the treatment of cancer or others diseases treatable with these drugs.
Nanoparticles of 4,7-dichloro-2-quinolinemethylacrylate-based copolymers and their potential cytotoxic activity on human breast carcinoma cells
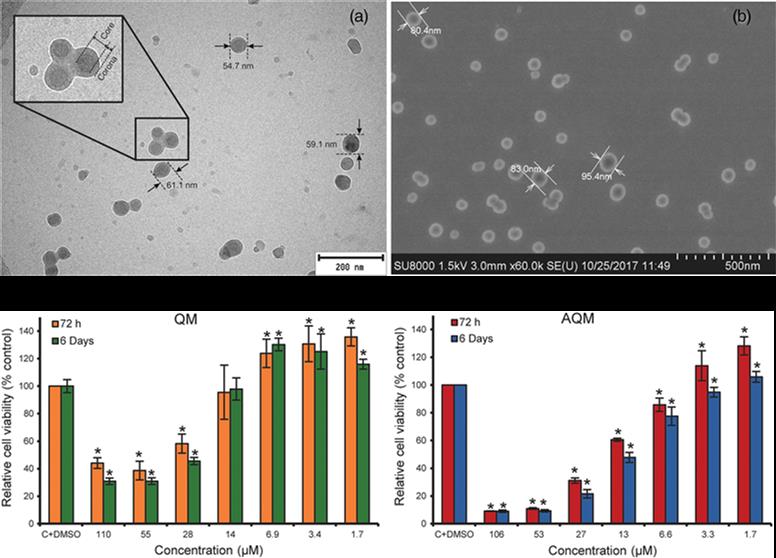
In this article, an improved synthesis strategy of the potent anticancer compound 4,7-dichloro-2-quinolinemethanol (QM) and its acrylate ester 4,7-dichloro-2-quinolinemethylacrylate (AQM) are described. AQM is copolymerized using free-radical polymerization with N-vinyl-2-pyrrolidone (VP) and the copolymers obtained from different molar ratios of monomers are subjected to nanoprecipitation to produce suspensions of nanoparticles (NPs) in phosphate buffered saline (PBS). The smallest and stable NPs are
prepared with the AQM-VP copolymers 45:55 and 40:60 (118.9 and 128.7 nm in diameter, respectively) at 1 mg mL−1, and along with AQM and QM, are evaluated for their cytotoxic activity on MDA-MB-453 breast carcinoma cells using MTT bioassay. AQM and QM are highly cytotoxic (IC50: 19 and 41 μM, respectively); however, the NPs are not cytotoxic in the range of the assayed concentrations. These results contribute to the search for new polymeric NPs with potential application as QM delivery systems for the treatment of cancer or other diseases treatable with QM.
1.Valle, H. et al. Nanoparticles of 4,7-dichloro-2-quinolinemethylacrylate-based copolymers and their potential cytotoxic activity on human breast carcinoma cells. Journal of Applied Polymer Science 136, 47545 (2019). Cite