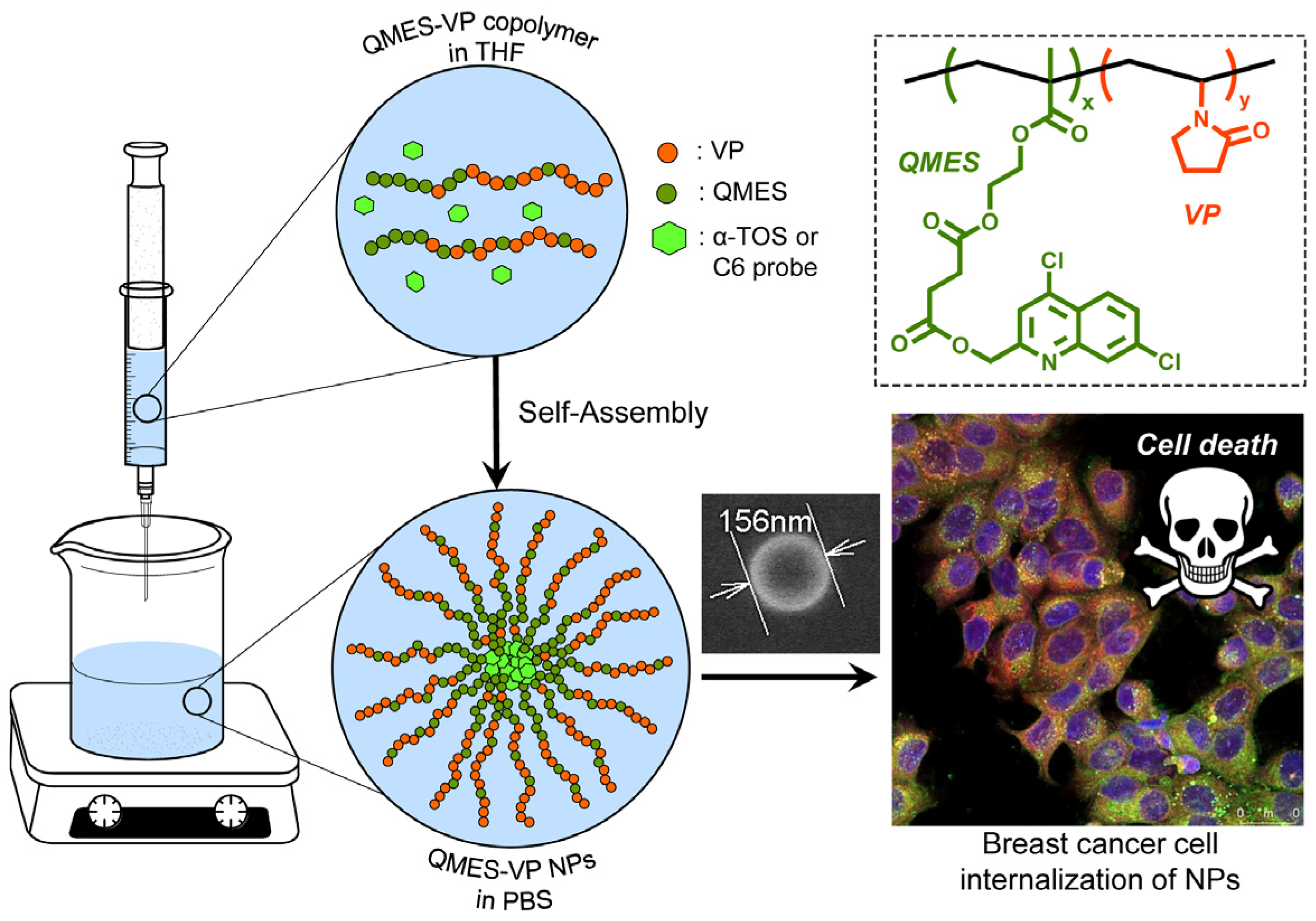
In order to improve the water solubility and, therefore, bioavailability and therapeutic activity of anticancer hydrophobic drug α-tocopherol succinate (α-TOS), in this work, copolymers were synthesized via free radicals from QMES (1-[4,7-dichloroquinolin-2-ylmethyl]-4-methacryloyloxyethyl succinate) and VP (N-vinyl-2-pirrolidone) using different molar ratios, and were used to nanoencapsulate and deliver α-TOS into cancer cells MCF-7. QMES monomer was chosen because the QMES pendant group in the polymer tends to hydrolyze to form free 4,7-dichloro-2-quinolinemethanol (QOH), which also, like α-TOS, exhibit anti-proliferative effects on cancerous cells. From the QMES-VP 30:70 (QMES-30) and 40:60 (QMES-40) copolymers obtained, it was possible to prepare aqueous suspensions of empty nanoparticles (NPs) loaded with α-TOS by nanoprecipitation. The diameter and encapsulation efficiency (%EE) of the QMES-30 NPs loaded with α-TOS were 128.6 nm and 52%; while for the QMES-40 NPs loaded with α-TOS, they were 148.8 nm and 65%. The results of the AlamarBlue assay at 72 h of treatment show that empty QMES-30 NPs (without α-TOS) produced a marked cytotoxic effect on MCF-7 breast cancer cells, corresponding to an IC50 value of 0.043 mg mL−1, and importantly, they did not exhibit cytotoxicity against healthy HUVEC cells. Furthermore, NP-QMES-40 loaded with α-TOS were cytotoxic with an IC50 value of 0.076 mg mL−1, demonstrating a progressive release of α-TOS; however, the latter nanoparticles were also cytotoxic to healthy cells in the range of the assayed concentrations. These results contribute to the search for a new polymeric nanocarrier of QOH, α-TOS or other hydrophobic drugs for the treatment of cancer or others diseases treatable with these drugs.
Ketoprofen-based polymer-drug nanoparticles provide anti-inf lammatory properties to HA/collagen hydrogels
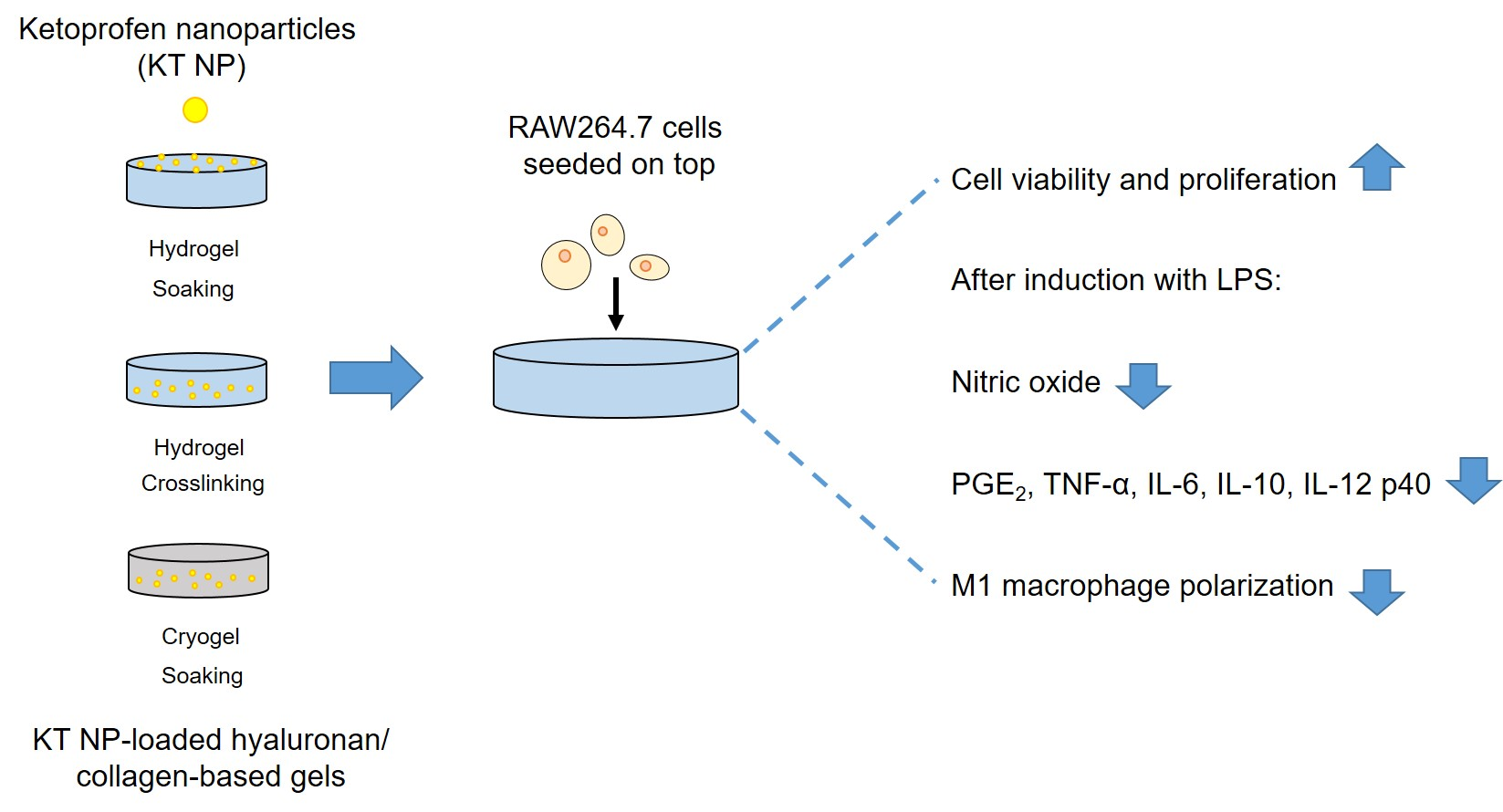
Current limitations of wound dressings for treating chronic wounds require the development of novel approaches. One of these is the immune-centered approach, which aims to restore the pro-regenerative and anti-inflammatory properties of macrophages. Under inflammatory conditions, ketoprofen nanoparticles (KT NP) can reduce pro-inflammatory markers of macrophages and increase anti-inflammatory cytokines. To assess their suitability as part of wound dressings, these NP were combined with hyaluronan (HA)/collagen-based hydro- (HG) and cryogels (CG). Different HA, NP concentrations and loading techniques for NP incorporation were used. The NP release, gel morphology and mechanical properties were studied. Generally, colonialization of the gels with macrophages resulted in high cell viability and proliferation. Furthermore, direct contact of the NP to the cells reduced the level of nitric oxide (NO). The formation of multinucleated cells on the gels was low and further decreased by the NP. For the HG that produced the highest reduction in NO, extended ELISA studies showed reduced levels of the pro-inflammatory markers PGE2, IL-12 p40, TNF-α, and IL-6. Thus, HA/collagen-based gels con-taining KT NP may represent a novel therapeutic approach for treating chronic wounds. Whether effects observed in vitro translate into a favorable profile on skin regeneration in vivo will require rigorous testing.
DEAE/Catechol-Chitosan Conjugates as Bioactive Polymers: Synthesis, Characterization, and Potential Applications
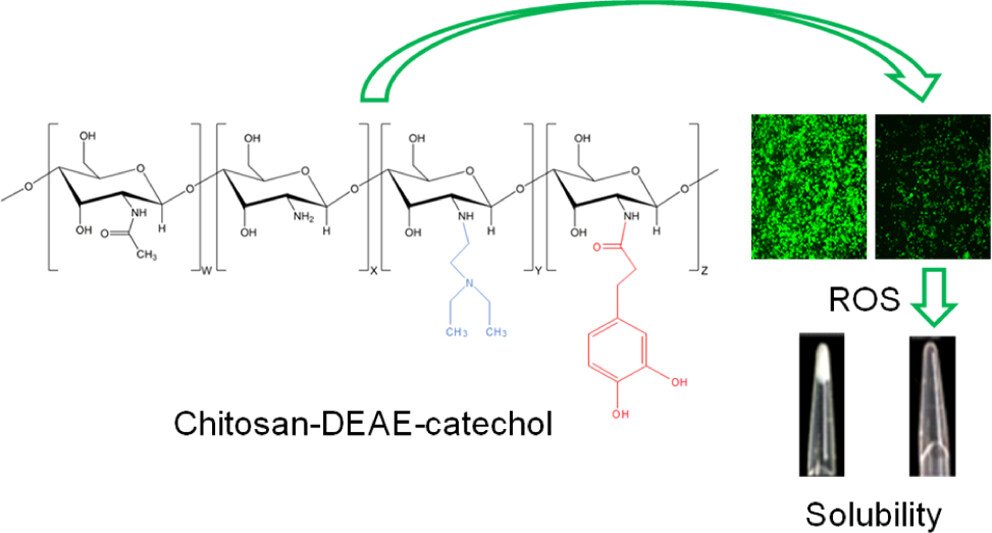
This work provides the first description of the synthesis and characterization of water-soluble chitosan (Cs) derivatives based on the conjugation of both diethylaminoethyl (DEAE) and catechol groups onto the Cs backbone (Cs–DC) in order to obtain a Cs derivative with antioxidant and antimicrobial properties. The degree of substitution [DS (%)] was 35.46% for DEAE and 2.53% for catechol, determined by spectroscopy. Changes in the molecular packing due to the incorporation of both pendant groups were described by X-ray diffraction and thermogravimetric analysis. For Cs, the crystallinity index was 59.46% and the maximum decomposition rate appeared at 309.3 °C, while for Cs–DC, the values corresponded to 16.98% and 236.4 °C, respectively. The incorporation of DEAE and catechol groups also increases the solubility of the polymer at pH > 7 without harming the antimicrobial activity displayed by the unmodified polymer. The catecholic derivatives increase the radical scavenging activity in terms of the half-maximum effective concentration (EC50). An EC50 of 1.20 μg/mL was found for neat hydrocaffeic acid (HCA) solution, while for chitosan–catechol (Cs–Ca) and Cs–DC solutions, concentrations equivalent to free HCA of 0.33 and 0.41 μg/mL were required, respectively. Cell culture results show that all Cs derivatives have low cytotoxicity, and Cs–DC showed the ability to reduce the activity of reactive oxygen species by 40% at concentrations as low as 4 μg/mL. Polymeric nanoparticles of Cs derivatives with a hydrodynamic diameter (Dh) of around 200 nm, unimodal size distributions, and a negative ζ-potential were obtained by ionotropic gelation and coated with hyaluronic acid in aqueous suspension, providing the multifunctional nanoparticles with higher stability and a narrower size distribution.
DEAE-chitosan nanoparticles as a pneumococcus-biomimetic material for the development of antipneumococcal therapeutics
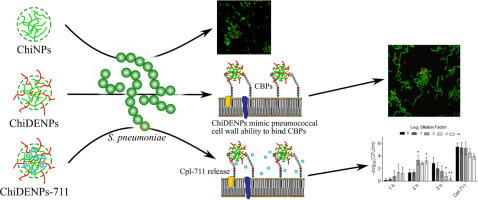
Advanced biomaterials provide an interesting and versatile platform to implement new and more effective strategies to fight bacterial infections. Chitosan is one of these biopolymers and possesses relevant features for biomedical applications. Here we synthesized nanoparticles of chitosan derivatized with diethylaminoethyl groups (ChiDENPs) to emulate the choline residues in the pneumococcal cell wall and act as ligands for choline-binding proteins (CBPs). Firstly, we assessed the ability of diethylaminoethyl (DEAE) to sequester the CBPs present in the bacterial surface, thus promoting chain formation. Secondly, the CBP-binding ability of ChiDENPs was purposed to encapsulate a bio-active molecule, the antimicrobial enzyme Cpl-711 (ChiDENPs-711), with improved stability over non-derivatized chitosan. The enzyme-loaded system released more than 90% of the active enzybiotic in ≈ 2 h, above the usual in vivo half-life of this kind of enzymes. Therefore, ChiDENPs provide a promising platform for the controlled release of CBP-enzybiotics in biological contexts.
Modulation of Inflammatory Mediators by Polymeric Nanoparticles Loaded with Anti-Inflammatory Drugs
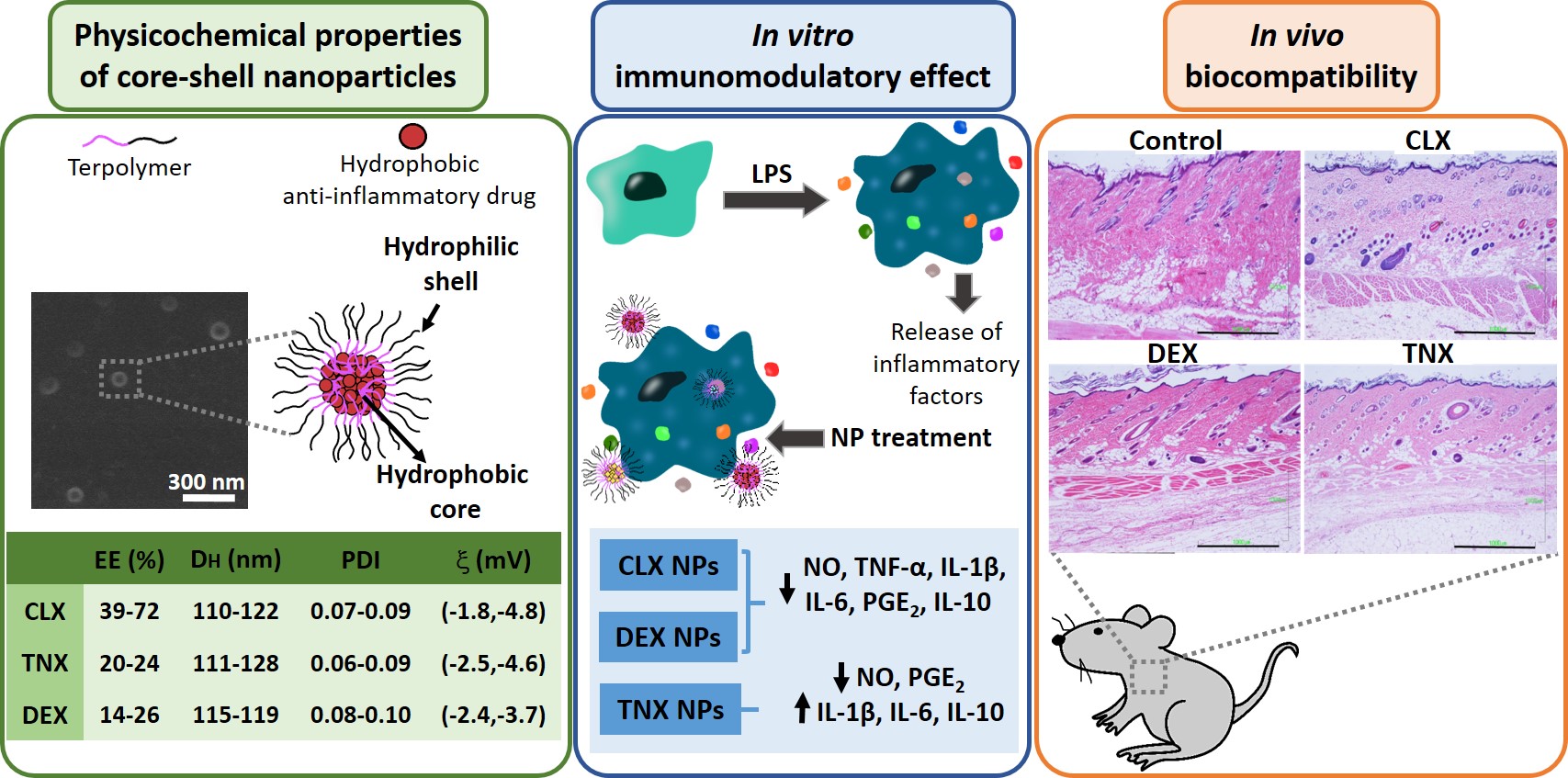
The first-line treatment of osteoarthritis is based on anti-inflammatory drugs, the most currently used being nonsteroidal anti-inflammatory drugs, selective cyclooxygenase 2 (COX-2) inhibitors and corticoids. Most of them present cytotoxicity and low bioavailabil-ity in physiological conditions, making necessary the administration of high drug con-centrations causing several side effects. The goal of this work was to encapsulate three hydrophobic anti-inflammatory drugs of different natures (celecoxib, tenoxicam and dexamethasone) into core-shell terpolymer nanoparticles with potential applications in osteoarthritis. Nanoparticles presented hydrodynamic diameters between 110 and 130 nm and almost neutral surface charges (between −1 and −5 mV). Encapsulation efficien-cies were highly dependent on the loaded drug and its water solubility, having higher values for celecoxib (39–72%) followed by tenoxicam (20–24%) and dexamethasone (14–26%). Nanoencapsulation reduced celecoxib and dexamethasone cytotoxicity in human articular chondrocytes and murine RAW264.7 macrophages. Moreover, the three loaded systems did not show cytotoxic effects in a wide range of concentrations. Celecoxib and dexamethasone-loaded nanoparticles reduced the release of different inflammatory medi-ators (NO, TNF-α, IL-1β, IL-6, PGE2 and IL-10) by lipopolysaccharide (LPS)-stimulated RAW264.7. Tenoxicam-loaded nanoparticles reduced NO and PGE2 production, although an overexpression of IL-1β, IL-6 and IL-10 was observed. Finally, all nanoparticles proved to be biocompatible in a subcutaneous injection model in rats. These findings suggest that these loaded nanoparticles could be suitable candidates for the treatment of inflammatory processes associated with osteoarthritis due to their demonstrated in vitro activity as reg-ulators of inflammatory mediator production.
Amphiphilic polymeric nanoparticles encapsulating curcumin: Antioxidant, anti-inflammatory and biocompatibility studies
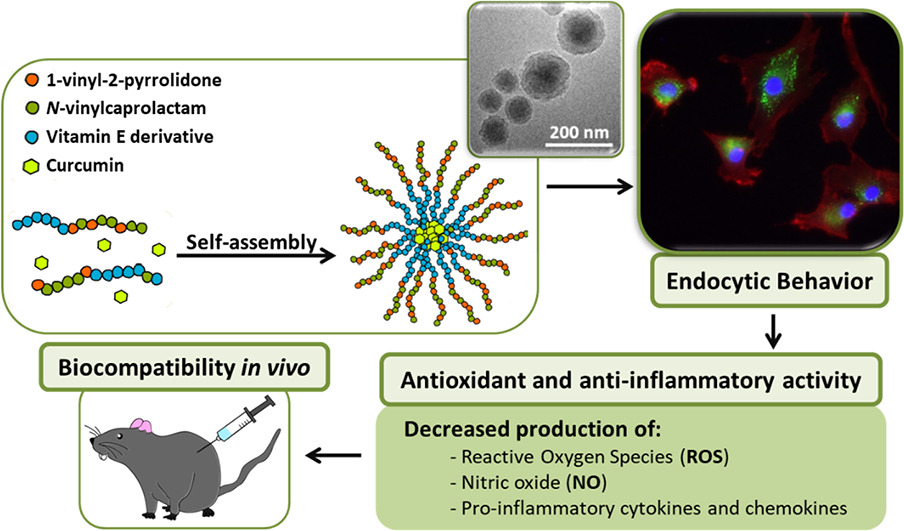
Oxidative stress and inflammation are two related processes common to many diseases. Curcumin is a natural compound with both antioxidant and anti-inflammatory properties, among others, that is recently being used as a natural occurring product alternative to traditional drugs. However, it has a hydrophobic nature that compromises its solubility in physiological fluids and its circulation time and also presents cytotoxicity problems in its free form, limiting the range of concentrations to be used. In order to overcome these drawbacks and taking advantage of the benefits of nanotechnology, the aim of this work is the development of curcumin loaded polymeric nanoparticles that can provide a controlled release of the drug and enlarge their application in the treatment of inflammatory and oxidative stress related diseases. Specifically, the vehicle is a bioactive terpolymer based on a α-tocopheryl methacrylate, 1-vinyl-2-pyrrolidone and N-vinylcaprolactam. Nanoparticles were obtained by nanoprecipitation and characterized in terms of size, morphology, stability, encapsulation efficiency and drug release. In vitro cellular assays were performed in human articular chondrocyte and RAW 264.7 cultures to assess cytotoxicity, cellular uptake, antioxidant and anti-inflammatory properties. The radical scavenging activity of the systems was confirmed by the DPPH test and the quantification of cellular reactive oxygen species. The anti-inflammatory potential of these systems was demonstrated by the reduction of different pro-inflammatory factors such as IL-8, MCP and MIP in chondrocytes; and nitric oxide, IL-6, TNF-α and MCP-1, among others, in RAW 264.7. Finally, the in vivo biocompatibility was confirmed in a rat model by subcutaneously injecting the nanoparticle dispersions. The reduction of curcumin toxicity and the antioxidant, anti-inflammatory and biocompatibility properties open the door to deeper in vitro and in vivo research on these curcumin loaded polymeric nanoparticles to treat inflammation and oxidative stress based diseases.
Nanoparticles of 4,7-dichloro-2-quinolinemethylacrylate-based copolymers and their potential cytotoxic activity on human breast carcinoma cells
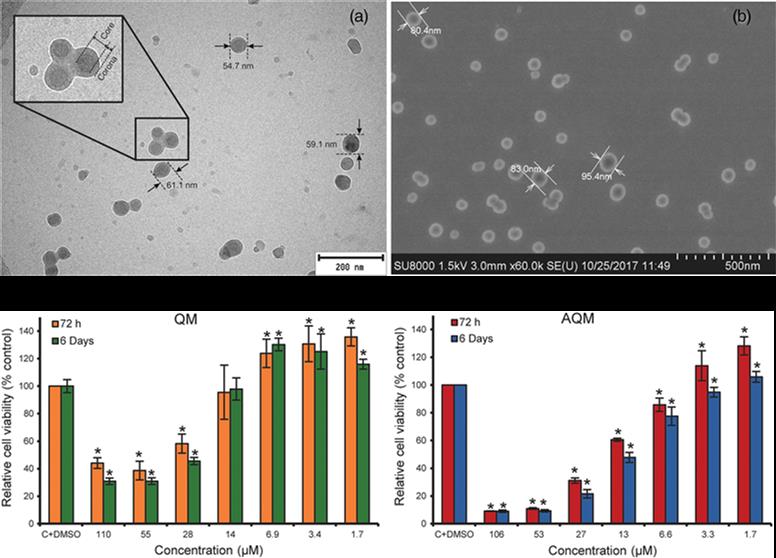
In this article, an improved synthesis strategy of the potent anticancer compound 4,7-dichloro-2-quinolinemethanol (QM) and its acrylate ester 4,7-dichloro-2-quinolinemethylacrylate (AQM) are described. AQM is copolymerized using free-radical polymerization with N-vinyl-2-pyrrolidone (VP) and the copolymers obtained from different molar ratios of monomers are subjected to nanoprecipitation to produce suspensions of nanoparticles (NPs) in phosphate buffered saline (PBS). The smallest and stable NPs are
prepared with the AQM-VP copolymers 45:55 and 40:60 (118.9 and 128.7 nm in diameter, respectively) at 1 mg mL−1, and along with AQM and QM, are evaluated for their cytotoxic activity on MDA-MB-453 breast carcinoma cells using MTT bioassay. AQM and QM are highly cytotoxic (IC50: 19 and 41 μM, respectively); however, the NPs are not cytotoxic in the range of the assayed concentrations. These results contribute to the search for new polymeric NPs with potential application as QM delivery systems for the treatment of cancer or other diseases treatable with QM.
1.Valle, H. et al. Nanoparticles of 4,7-dichloro-2-quinolinemethylacrylate-based copolymers and their potential cytotoxic activity on human breast carcinoma cells. Journal of Applied Polymer Science 136, 47545 (2019). Cite
pH-sensitive polymeric nanoparticles with antioxidant and anti-inflammatory properties against cisplatin-induced hearing loss
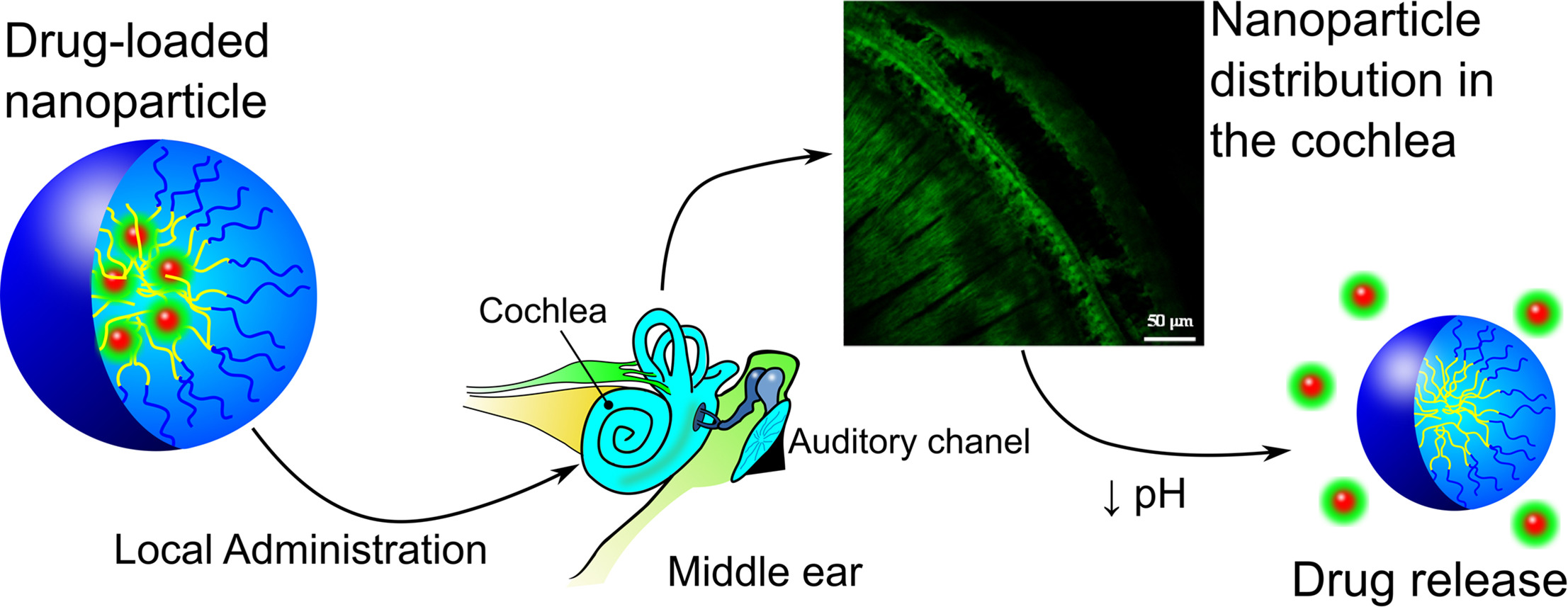
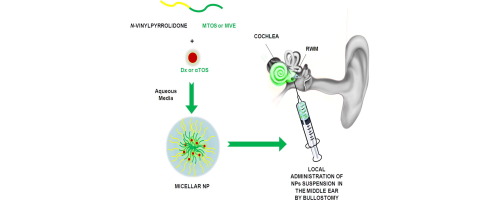
Polymeric nanoparticles (NP) based on smart synthetic amphiphilic copolymers are used to transport and controlled release dexamethasone in the inner ear to protect against the ototoxic effect of cisplatin. The NP were based on a mixture of two pseudo-block polymer drugs obtained by free radical polymerization: poly(VI-co-HEI) and poly(VP-co-MVE) or poly(VP-co-MTOS), being VI 1-vinylimidazole, VP N-vinylpyrrolidone, and IBU, MVE and MTOS the methacrylic derivatives of ibuprofen, α-tocopherol and α-tocopheryl succinate, respectively. The NP were obtained by nanoprecipitation with appropriate hydrodynamic properties, and isoelectric points that matched the pH of inflamed tissue. The NP were tested both in vitro (using HEI-OC1 cells) and in vivo (using a murine model) with good results. Although the concentration of dexamethasone administered in the nanoparticles is around two orders of magnitude lower that the conventional treatment for intratympanic administration, the NP protected from the cytotoxic effect of cisplatin when the combination of the appropriate properties in terms of size, zeta potential, encapsulation efficiency and isoelectric point were achieved. To the best of our knowledge this is the first time that pH sensitive NP are used to protect from cisplatin-induced hearing loss by intratympanic administration.
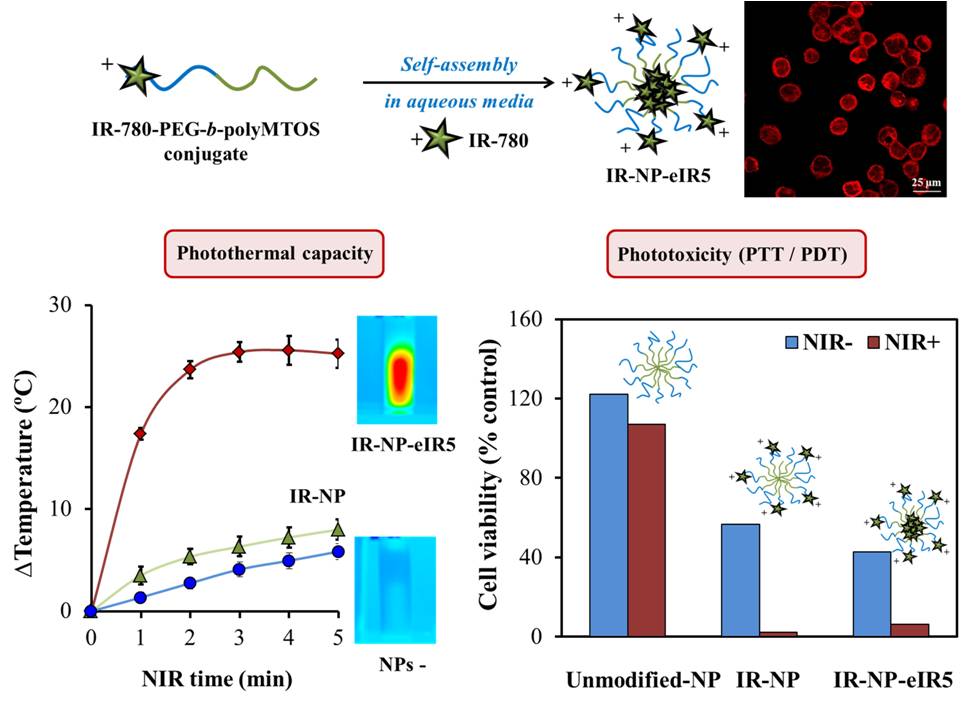
Photothermal and photodynamic activity of polymeric nanoparticles based on α-tocopheryl succinate-RAFT block copolymers conjugated to IR-780
The aim of this work was the generation of a multifunctional nanopolymeric system that incorporates IR- 780 dye, a near-infrared (NIR) imaging probe that exhibits photothermal and photodynamic properties; and a derivate of α-tocopheryl succinate (α-TOS), a mitochondria-targeted anticancer compound. IR-780 was conjugated to the hydrophilic segment of copolymer PEG-b-polyMTOS, based on poly(ethylene glycol) (PEG) and a methacrylic derivative of α-TOS (MTOS), to generate IR-NP, selfassembled nanoparticles (NPs) in aqueous media which exhibit a hydrophilic shell and a hydrophobic core. During assembly, the hydrophobic core of IR-NP could encapsulate additional IR-780 to generate derived subspecies carrying different amount of probe (IR-NP-eIR).
Evaluation of photo-inducible properties of IR-NP and IR-NP-eIR were thoroughly assessed in vitro. Developed nanotheranostic particles showed distinct fluorescence and photothermal behavior after excitation by a laser light emitting at 808 nm. Treatment of MDA-MB-453 cells with IR-NP or IR-NP-eIR resulted in an efficient internalization of the IR-780 dye, while subsequent NIR-laser irradiation led to a severe decrease in cell viability. Photocytoxicity conducted by IR-NP, which could not be attributed to the generation of lethal hyperthermia, responded to an increase in the levels of intracellular reactive oxygen species (ROS). Therefore, the fluorescence imaging and inducible phototoxicity capabilities of NPs derived from IR-780-PEG-bpolyMTOS copolymer confer high value to these nanotheranostics tools in clinical cancer research.
Polymeric nanoparticles loaded with dexamethasone or α-tocopheryl succinate to prevent cisplatin-induced ototoxicity
The aim of this work is the development of highly protective agents to be administered locally within the middle ear to avoid cisplatin-induced ototoxicity, which affects to 100% of the clinical patients at ultrahigh concentrations (16 mg/kg). The protective agents are based on polymeric nanoparticles loaded with dexamethasone or α-tocopheryl succinate as anti-inflammarory and anti-apoptotic molecules.
Dexamethasone and α-tocopheryl succinate are poorly soluble in water and present severe side effects when systemic administered during long periods of time. Their incorporation in the hydrophobic core of nanoparticles with the appropriate hydrodynamic properties provides the desired effects in vitro (lower cisplatin-induced toxicity, decreasing of caspase 3/7 activity, and lower IL-1b release) and in vivo (reducing the hearing loss at the local level). The local administration of the nanoparticles by bullostomy provides an adequate dose of drug without systemic interference with the chemotherapeutic effect of cisplatin.