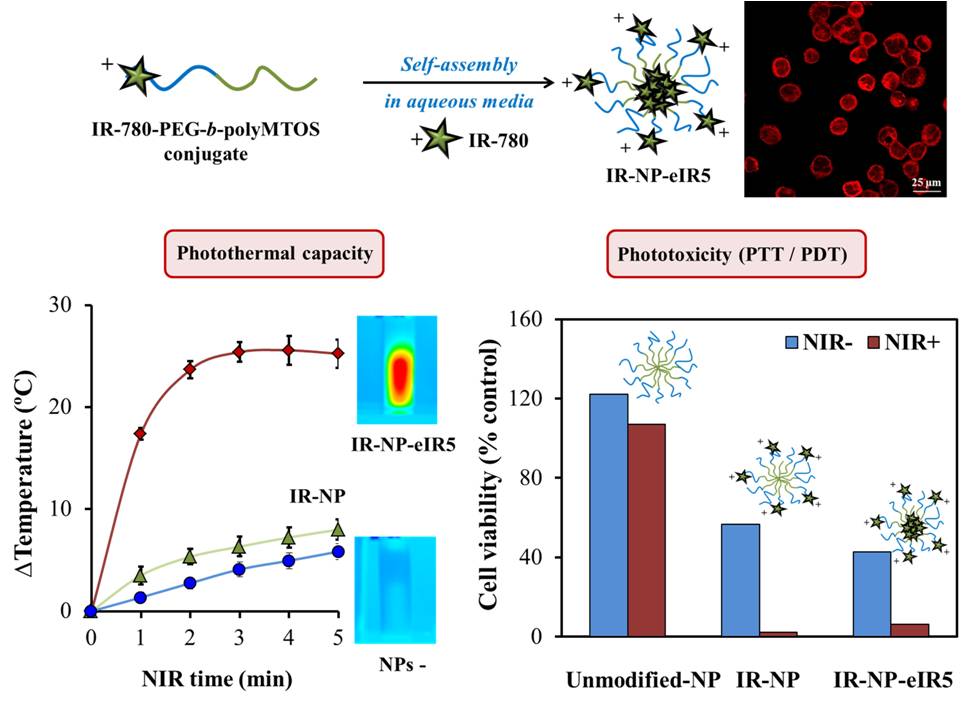
The aim of this work was the generation of a multifunctional nanopolymeric system that incorporates IR- 780 dye, a near-infrared (NIR) imaging probe that exhibits photothermal and photodynamic properties; and a derivate of α-tocopheryl succinate (α-TOS), a mitochondria-targeted anticancer compound. IR-780 was conjugated to the hydrophilic segment of copolymer PEG-b-polyMTOS, based on poly(ethylene glycol) (PEG) and a methacrylic derivative of α-TOS (MTOS), to generate IR-NP, selfassembled nanoparticles (NPs) in aqueous media which exhibit a hydrophilic shell and a hydrophobic core. During assembly, the hydrophobic core of IR-NP could encapsulate additional IR-780 to generate derived subspecies carrying different amount of probe (IR-NP-eIR).
Evaluation of photo-inducible properties of IR-NP and IR-NP-eIR were thoroughly assessed in vitro. Developed nanotheranostic particles showed distinct fluorescence and photothermal behavior after excitation by a laser light emitting at 808 nm. Treatment of MDA-MB-453 cells with IR-NP or IR-NP-eIR resulted in an efficient internalization of the IR-780 dye, while subsequent NIR-laser irradiation led to a severe decrease in cell viability. Photocytoxicity conducted by IR-NP, which could not be attributed to the generation of lethal hyperthermia, responded to an increase in the levels of intracellular reactive oxygen species (ROS). Therefore, the fluorescence imaging and inducible phototoxicity capabilities of NPs derived from IR-780-PEG-bpolyMTOS copolymer confer high value to these nanotheranostics tools in clinical cancer research.