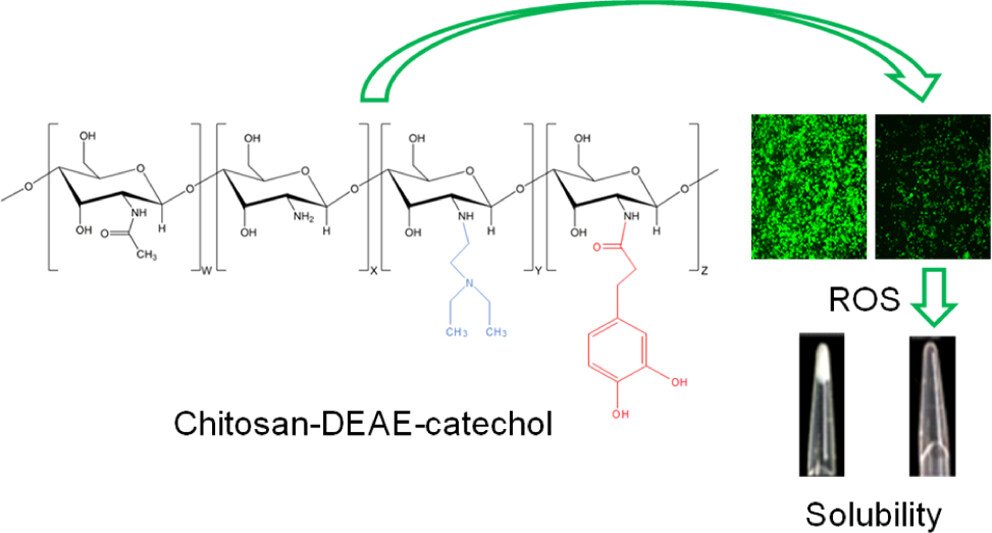
This work provides the first description of the synthesis and characterization of water-soluble chitosan (Cs) derivatives based on the conjugation of both diethylaminoethyl (DEAE) and catechol groups onto the Cs backbone (Cs–DC) in order to obtain a Cs derivative with antioxidant and antimicrobial properties. The degree of substitution [DS (%)] was 35.46% for DEAE and 2.53% for catechol, determined by spectroscopy. Changes in the molecular packing due to the incorporation of both pendant groups were described by X-ray diffraction and thermogravimetric analysis. For Cs, the crystallinity index was 59.46% and the maximum decomposition rate appeared at 309.3 °C, while for Cs–DC, the values corresponded to 16.98% and 236.4 °C, respectively. The incorporation of DEAE and catechol groups also increases the solubility of the polymer at pH > 7 without harming the antimicrobial activity displayed by the unmodified polymer. The catecholic derivatives increase the radical scavenging activity in terms of the half-maximum effective concentration (EC50). An EC50 of 1.20 μg/mL was found for neat hydrocaffeic acid (HCA) solution, while for chitosan–catechol (Cs–Ca) and Cs–DC solutions, concentrations equivalent to free HCA of 0.33 and 0.41 μg/mL were required, respectively. Cell culture results show that all Cs derivatives have low cytotoxicity, and Cs–DC showed the ability to reduce the activity of reactive oxygen species by 40% at concentrations as low as 4 μg/mL. Polymeric nanoparticles of Cs derivatives with a hydrodynamic diameter (Dh) of around 200 nm, unimodal size distributions, and a negative ζ-potential were obtained by ionotropic gelation and coated with hyaluronic acid in aqueous suspension, providing the multifunctional nanoparticles with higher stability and a narrower size distribution.
3729048
{3729048:KGTEP5EW}
1
nature
50
default
1
1179
http://www.biomateriales.ictp.csic.es/wp-content/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22KGTEP5EW%22%2C%22library%22%3A%7B%22id%22%3A3729048%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Caro-Le%5Cu00f3n%20Fj%20et%20al.%22%2C%22parsedDate%22%3A%222023-02-13%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ECaro-Le%26%23xF3%3Bn%20Fj%20%3Ci%3Eet%20al.%3C%5C%2Fi%3E%20DEAE%5C%2FCatechol-Chitosan%20Conjugates%20as%20Bioactive%20Polymers%3A%20Synthesis%2C%20Characterization%2C%20and%20Potential%20Applications.%20%3Ci%3EBiomacromolecules%3C%5C%2Fi%3E%20%3Cb%3E24%3C%5C%2Fb%3E%2C%20%282023%29.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22DEAE%5C%2FCatechol-Chitosan%20Conjugates%20as%20Bioactive%20Polymers%3A%20Synthesis%2C%20Characterization%2C%20and%20Potential%20Applications%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Caro-Le%5Cu00f3n%20Fj%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22L%5Cu00f3pez-Donaire%20Ml%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22V%5Cu00e1zquez%20R%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Huerta-Madro%5Cu00f1al%20M%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Lizardi-Mendoza%20J%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Arg%5Cu00fcelles-Monal%20Wm%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Fern%5Cu00e1ndez-Quiroz%20D%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Garc%5Cu00eda-Fern%5Cu00e1ndez%20L%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22San%20Roman%20J%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22V%5Cu00e1zquez-Lasa%20B%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Garc%5Cu00eda%20P%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Aguilar%20Mr%22%7D%5D%2C%22abstractNote%22%3A%22This%20work%20provides%20the%20first%20description%20of%20the%20synthesis%20and%20characterization%20of%20water-soluble%20chitosan%20%28Cs%29%20derivatives%20based%20on%20the%20conjugation%20of%20both%20diethylaminoethyl%20%28DEAE%29%20and%20catechol%20groups%20onto%20the%20Cs%20backbone%20%28Cs-DC%29%20in%20order%20to%20obtain%20a%20Cs%20derivative%20with%20antioxidant%20and%20antimicrobial%20p%20%5Cu2026%22%2C%22date%22%3A%2202%5C%2F13%5C%2F2023%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1021%5C%2Facs.biomac.2c01012%22%2C%22ISSN%22%3A%221526-4602%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fpubmed.ncbi.nlm.nih.gov%5C%2F36594453%5C%2F%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222023-03-09T12%3A18%3A01Z%22%7D%7D%5D%7D
1.
Caro-León Fj et al. DEAE/Catechol-Chitosan Conjugates as Bioactive Polymers: Synthesis, Characterization, and Potential Applications. Biomacromolecules 24, (2023).