Immune response to biomaterials can produce chronic inflammation and fibrosis leading to implant failure, which is related to the surface properties of the biomaterials. This work describes the preparation and characterization of polyelectrolyte multilayer (PEM) coatings that combine the anti-inflammatory activity of heparin as polyanion with the potential release of Naproxen, a nonsteroidal anti-inflammatory drug from polymeric nanoparticles (NP) with cationic surface charge. The polyelectrolyte multilayers were characterized by physical methods to estimate multilayer growth, thickness, zeta potential, and topography. It was found that multilayers with NP had negative zeta potentials and
expressed a viscoelastic behavior, while studies of topography showed that nanoparticles formed continuous surface coatings. THP-1-derived macrophages were used to study short-term antiinflammatory activity (time scale 48 h), showing that PEM that contained heparin reduced cell adhesion and IL1-β secretion, when compared to those with polystyrenesulfonate, used as alternative polyanion in multilayer formation. On the other hand, the presence of NP in PEM was related to a reduced foreign body giant cell formation after 15 days, when compared to PEM that contained chitosan as alternative polycation, which suggests a long-term anti-inflammatory effect of Naproxen-containing nanoparticles. It was also shown that macrophages were able to take up NP from multilayers, which indicates a release of Naproxen by digestion of NP in the lysosomal compartment. These findings indicate that surface coatings composed of heparin and Naproxen-based NP on implants such as biosensors have the potential to attenuate foreign body reaction after implantation, which may improve the long-term functionality of implants.
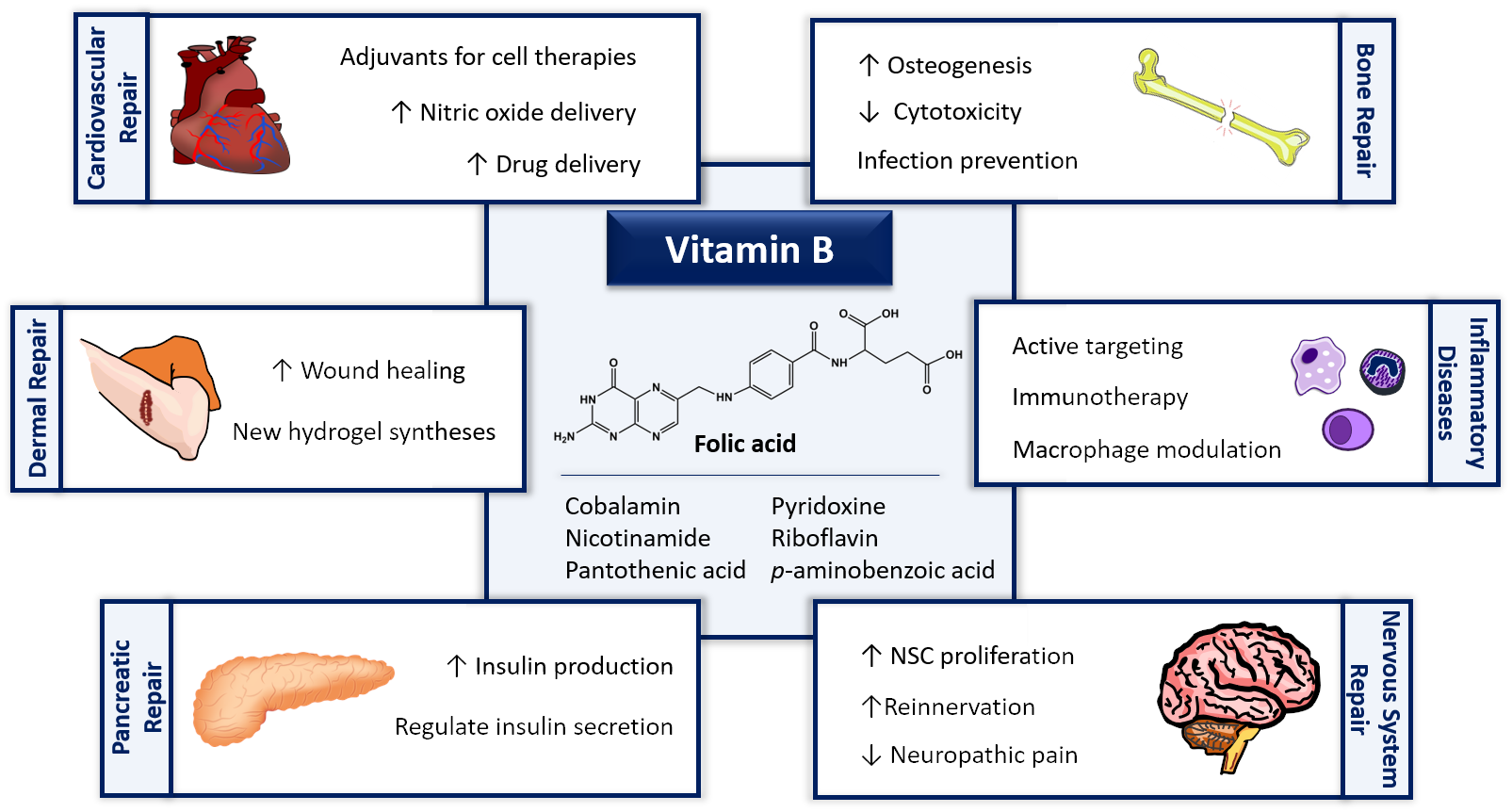
Tissue Engineering therapies based on folic acid and other vitamin B derivatives. Functional mechanisms and current applications in Regenerative Medicine.
B-vitamins are a group of soluble vitamins which are cofactors of some of the enzymes involved in the metabolic pathways of carbohydrates, fats and proteins. These compounds participate in a number of functions as cardiovascular, brain or nervous systems. Folic acid is described as an accessible and multifunctional niche component that can be used safely, even combined with other compounds, which gives it high versatility. Also, due to its non-toxicity and great stability, folic acid has attracted much attention from researchers in the biomedical and bioengineering area, with an increasing number of works directed at using folic acid and its derivatives in tissue engineering therapies as well as regenerative medicine. Thus, this review provides an updated discussion about the most relevant advances achieved during the last five years, where folic acid and other vitamins B have been used as key bioactive compounds for enhancing the effectiveness of biomaterials’ performance and biological functions for the regeneration of tissues and organs.
1.Fernández-Villa, D., Jiménez Gómez-Lavín, M., Abradelo, C., San Román, J. & Rojo, L. Tissue Engineering Therapies Based on Folic Acid and Other Vitamin B Derivatives. Functional Mechanisms and Current Applications in Regenerative Medicine. Int J Mol Sci 19, (2018). Cite
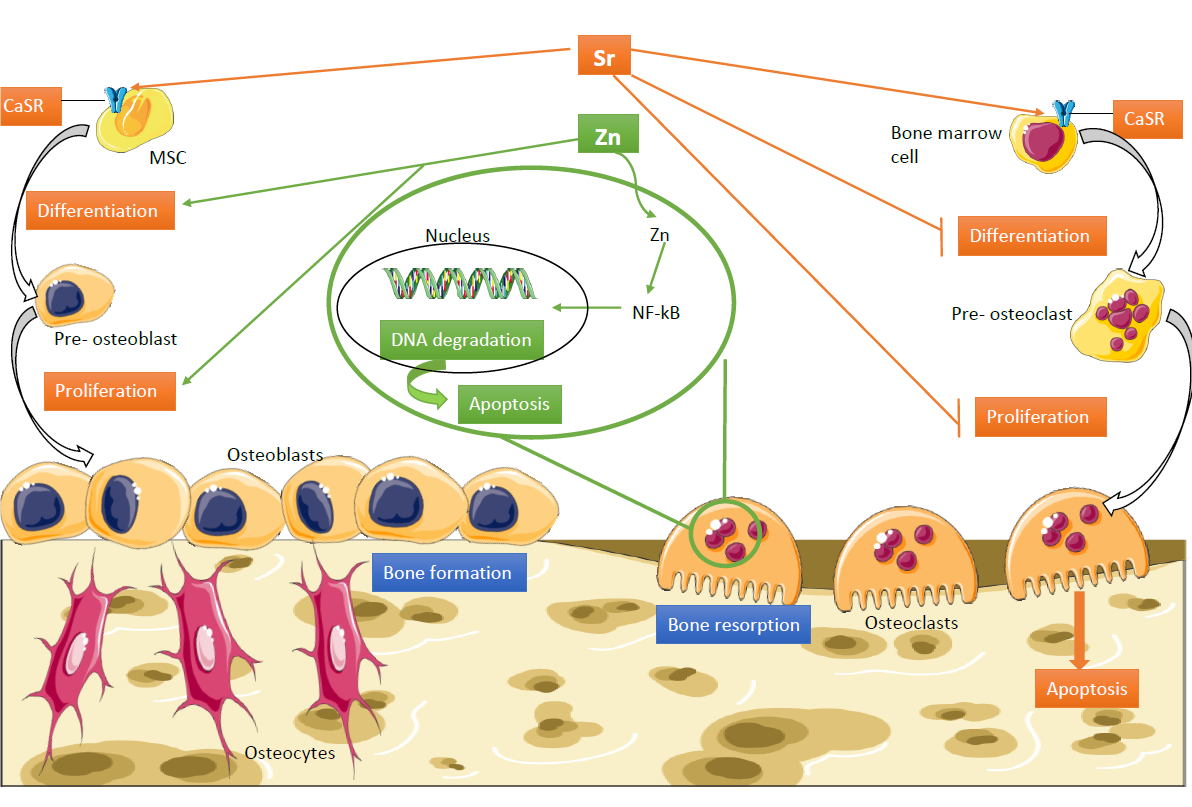
Bibliographic review on the state of the art of strontium and zinc based regenerative therapies. Recent developments and clinical applications
This review brings up to date the state of the art of strontium and zinc based regenerative therapies, both having a promoting effect on tissue formation and a role inhibiting resorption in musculoskeletal disorders.
1.Jiménez, M., Abradelo, C., Román, J. S. & Rojo, L. Bibliographic review on the state of the art of strontium and zinc based regenerative therapies. Recent developments and clinical applications. J. Mater. Chem. B (2019) http://doi.org/10.1039/C8TB02738B. Cite
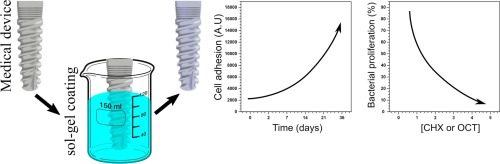
A single coating with antibacterial properties for prevention of medical device-associated infections
Human bacterial pathogens found on medical implants are one of the main causes of morbidity and mortality, making the development of novel coatings the primary strategy in the prevention of medical device-associated infections. Here, we describe organic-inorganic hybrid coatings for metallic bone implants, based on sol-gel materials of proven osteogenic capacity. The coatings were doped with two bactericides: octenidine dihydrochloride (OCT) and chlorhexidine diacetate (CHX). These bactericides, known for their efficiency, are widely used in the prevention and elimination of bacterial infections. The coatings have good chemical and mechanical properties, making them suitable for use on medical devices. They also demonstrate strong antibacterial capacity, dependent on the concentration of the bactericide. They are not toxic to human osteoblasts. Our results suggest this system as a tool for coating medical devices to prevent bacterial infections.
1.García-Arnáez, I. et al. A single coating with antibacterial properties for prevention of medical device-associated infections. European Polymer Journal 113, 289–296 (2019). Cite
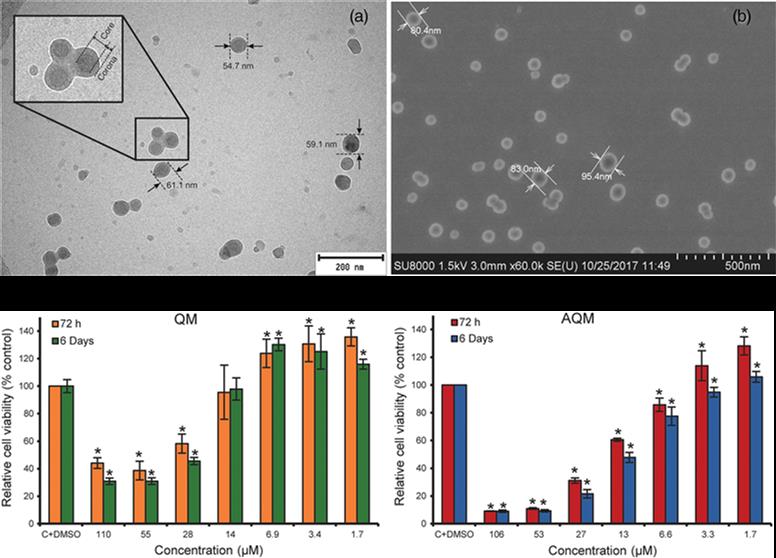
Nanoparticles of 4,7-dichloro-2-quinolinemethylacrylate-based copolymers and their potential cytotoxic activity on human breast carcinoma cells
In this article, an improved synthesis strategy of the potent anticancer compound 4,7-dichloro-2-quinolinemethanol (QM) and its acrylate ester 4,7-dichloro-2-quinolinemethylacrylate (AQM) are described. AQM is copolymerized using free-radical polymerization with N-vinyl-2-pyrrolidone (VP) and the copolymers obtained from different molar ratios of monomers are subjected to nanoprecipitation to produce suspensions of nanoparticles (NPs) in phosphate buffered saline (PBS). The smallest and stable NPs are
prepared with the AQM-VP copolymers 45:55 and 40:60 (118.9 and 128.7 nm in diameter, respectively) at 1 mg mL−1, and along with AQM and QM, are evaluated for their cytotoxic activity on MDA-MB-453 breast carcinoma cells using MTT bioassay. AQM and QM are highly cytotoxic (IC50: 19 and 41 μM, respectively); however, the NPs are not cytotoxic in the range of the assayed concentrations. These results contribute to the search for new polymeric NPs with potential application as QM delivery systems for the treatment of cancer or other diseases treatable with QM.
1.Valle, H. et al. Nanoparticles of 4,7-dichloro-2-quinolinemethylacrylate-based copolymers and their potential cytotoxic activity on human breast carcinoma cells. Journal of Applied Polymer Science 136, 47545 (2019). Cite
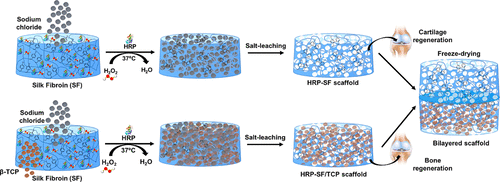
Enzymatically Cross-Linked Silk Fibroin-Based Hierarchical Scaffolds for Osteochondral Regeneration
Osteochondral (OC) regeneration faces several limitations in orthopedic surgery, owing to the complexity of the OC tissue that simultaneously entails the restoration of articular cartilage and subchondral bone diseases.
In this study, novel biofunctional hierarchical scaffolds composed of a horseradish peroxidase (HRP)-cross-linked silk fibroin (SF) cartilage-like layer (HRP-SF layer) fully integrated into a HRP-SF/ZnSr-doped β-tricalcium phosphate (β-TCP) subchondral bone-like layer (HRP-SF/dTCP layer) were proposed as a promising strategy for OC tissue regeneration. For comparative purposes, a similar bilayered structure produced with no ion incorporation (HRP-SF/TCP layer) was used. A homogeneous porosity distribution was achieved throughout the scaffolds, as shown by micro-computed tomography analysis. The ion-doped bilayered scaffolds presented a wet compressive modulus (226.56 ± 60.34 kPa) and dynamic mechanical properties (ranging from 403.56 ± 111.62 to 593.56 ± 206.90 kPa) superior to that of the control bilayered scaffolds (189.18 ± 90.80 kPa and ranging from 262.72 ± 59.92 to 347.68 ± 93.37 kPa, respectively). Apatite crystal formation, after immersion in simulated body fluid (SBF), was observed in the subchondral bone-like layers for the scaffolds incorporating TCP powders. Human osteoblasts (hOBs) and human articular chondrocytes (hACs) were co-cultured onto the bilayered structures and monocultured in the respective cartilage and subchondral bone half of the partitioned scaffolds. Both cell types showed good adhesion and proliferation in the scaffold compartments, as well as adequate integration of the interface regions. Osteoblasts produced a mineralized extracellular matrix (ECM) in the subchondral bone-like layers, and chondrocytes showed GAG deposition. The gene expression profile was different in the distinct zones of the bilayered constructs, and the intermediate regions showed pre-hypertrophic chondrocyte gene expression, especially on the BdTCP constructs. Immunofluorescence analysis supported these observations. This study showed that the proposed bilayered scaffolds allowed a specific stimulation of the chondrogenic and osteogenic cells in the co-culture system together with the formation of an osteochondral-like tissue interface. Hence, the structural adaptability, suitable mechanical properties, and biological performance of the hierarchical scaffolds make these constructs a desired strategy for OC defect regeneration.
1.Ribeiro, V. P. et al. Enzymatically Cross-Linked Silk Fibroin-Based Hierarchical Scaffolds for Osteochondral Regeneration. ACS Appl. Mater. Interfaces (2019) doi:10.1021/acsami.8b21259. Cite
α-Tocopheryl Succinate-Based Polymeric Nanoparticles for the Treatment of Head and Neck Squamous Cell Carcinoma
The aim of this work is to study, in an in vitro head and neck squamous cell carcinomas model the anti-angiogenic and anti-migratory properties of self-assembled polymeric nanoparticles (NPs) with demonstrated selective anticancer activity. The NPs are based on α-tocopheryl succinate (α-TOS) encapsulated in the hydrophobic core of the NPs. We analyzed the effect of the newly synthetized α-TOS-loaded NPs in proliferating endothelial cells and hypopharynx carcinoma squamous cells and measured markers of angiogenesis, apoptosis and reactive oxygen species (ROS). α-TOS-loaded NPs suppressed angiogenesis by inducing accumulation of ROS and inducing apoptosis of proliferating endothelial cells. These NPs also decrease the number and quality of capillary-like tubes in an in vitro three-dimensional (3D) experiment, decrease the production of the pro-angiogenic vascular endothelial growth factor and down-regulate the expression of its receptor. The anti-migratory efficacy of α-TOS is corroborated in hypopharynx carcinoma cells by decreasing the secretion of matrix metalloproteases 2 and 9 (MMP-2 and MMP-9) and inhibiting cell migration. These results confirm that α-TOS-based NPs not only present anticancer properties, but also antiangiogenic properties, therefore making them promising candidates for multi-active combinatorial anticancer therapy.
Polymeric Nanoparticles for Cancer Therapy and Bioimaging
Polymeric nanoparticles have demonstrated to be good candidates as vehicles of drugs or molecules for combined treatment and diagnosis of cancer. In comparison with inorganic nanoparticulated systems, they present remarkable benefits in terms of stability, biocompatibility, biodegradability, tailorability and low cost. Polymeric nanoparticles can be design to passively or actively accumulate in tumor sites by controlling their hydrodynamic properties or functionalizing their surface with targeting molecules. Moreover, polymers responding to particular tumor microenvironment conditions like reduced pH, high levels of reactive oxygen species or overexpressed enzymes, can be used to trigger a controlled drug delivery, a contrast agent exposure, or to enhance the therapeutic effect of a theranostic system.
This chapter focuses on the most recent advances in this field by discussing in depth examples of nanoparticles that, exploiting all these strategies, can be visualized with one or more imaging techniques: optical imaging, MRI, US, PA, PET, SPECT or CT; and present therapeutic effect (i.e. chemotherapy, gene therapy, photothermal or photodynamic therapy) due to the presence of active moieties.
In Situ Cross-Linkable Polymer Systems and Composites for Osteochondral Regeneration
Injectable hydrogels have demonstrated being a promising strategy for cartilage and bone tissue engineering applications, owing to their minimal invasive injection procedure, easy incorporation of cells and bioactive molecules, improved contact with the surrounding tissues and ability to match defects with complex irregular shapes, characteristics of osteoarthritic pathology. These unique properties make them highly suitable bioscaffolds for treating defects which are otherwise not easily accessible without and invasive surgical procedure. In this book chapter it has been summarized the novel appropriate injectable hydrogels for cartilage and bone tissue engineering applications of the last few years, including the most commonly used materials for the preparation, both natural and synthetic, and their fabrication techniques. The design of a suitable injectable hydrogel with an adequate gelation time that gathers perfect bioactive, biocompatible, biodegradable and good mechanical properties for clinical repair of damaged cartilage and bone tissue is a challenge of significant medical interest that remain to be achieved.
Osteochondral angiogenesis and promoted vascularization: New therapeutic target
The control of the different angiogenic process is an important point in osteochondral regeneration. Angiogenesis is a prerequisite for osteogenesis in vivo; insufficient neovascularization of bone constructs after scaffold implantation resulted in hypoxia and cellular necrosis. Otherwise, angiogenesis must be avoided in chondrogenesis; vascularization of the cartilage contributes to structural damage and pain. Finding a balance between these processes is important to design a successful treatment for osteochondral regeneration. This chapter shows the most important advances in the control of angiogenic process for the treatment of osteochondral diseases focused on the administration of pro- or anti-angiogenic factor and the design of the scaffold.