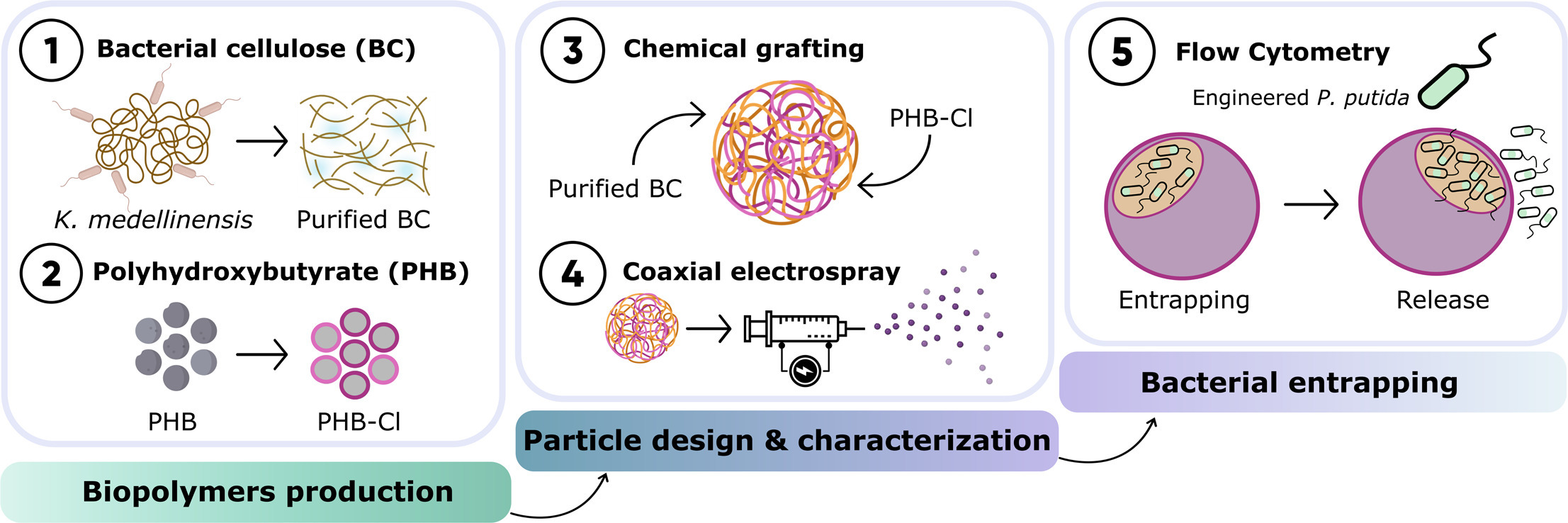
The design of biocarriers presents an effective approach for preserving bioactive elements and enabling controlled release in specific environments. This study introduces a novel biocarrier structure composed of two biodegradable, non-toxic, yet inherently incompatible bacterial biopolymers: bacterial cellulose (BC), a hydrophilic porous polymer known for its high water-holding capacity (up to 400 times its dry weight) and tensile strength, and polyhydroxybutyrate (PHB), a hydrophobic polymer characterized by its excellent barrier properties and UV stability. Using a coaxial electrospray technique, double-shelled hollow particles (DSHP) with a spherical architecture and an average diameter of 360 µm were produced. These particles consist of an outer PHB shell that serves as a protective barrier, and an inner BC-based layer designed to support microbial viability. To ensure structural integrity and enhance compatibility between the polymers, PHB chains were grafted onto BC, achieving a modification degree of 31%, prior to electrospraying. The resulting DSHP demonstrated an internal cavity capable of housing bacterial loads up to 108 CFU/mL, maintaining cell viability for at least 2 days and enabling controlled release profile. Additionally, the optimized electrospray conditions ensured high reproducibility and stability. This promising particle configuration offers potential applicability across various fields, from biomedicine to environmental applications.
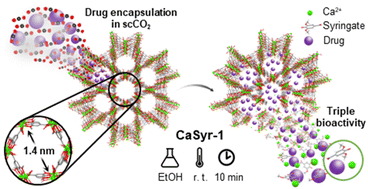
1.Rosado, A. et al. Supercritical CO2 assisted bioMOF drug encapsulation and functionalization for delivery with a synergetic therapeutic value. The Journal of Supercritical Fluids 106452 (2024) http://doi.org/10.1016/j.supflu.2024.106452. Cite