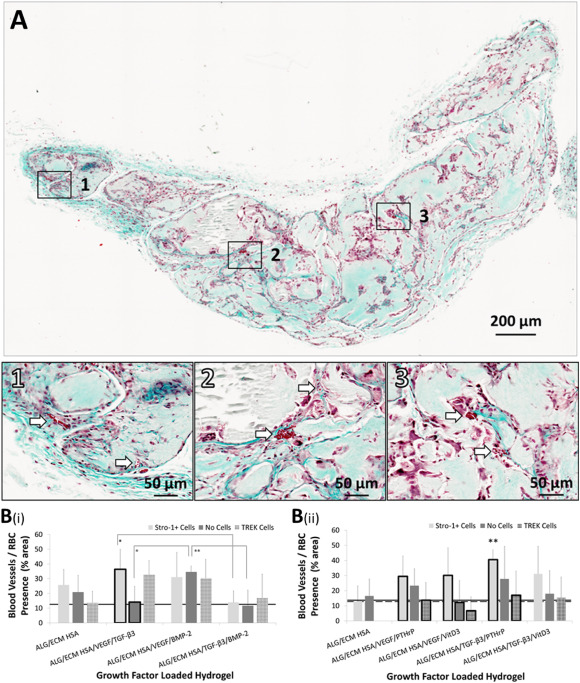
La ingeniería de tejidos óseos requiere una combinación de materiales, células, factores de crecimiento y estímulos mecánicos para replicar la formación ósea. En este estudio, evaluamos hidrogeles híbridos para la formación ósea mínimamente invasiva, combinando biomateriales con células madre esqueléticas y la liberación secuencial de factores de crecimiento junto con mecanotransducción. Los hidrogeles híbridos, compuestos de alginato y matriz extracelular ósea descelularizada y desmineralizada (ALG/ECM), fueron sembrados con células estromales de médula ósea humana Stro-1+ (HBMSCs). Se añadieron combinaciones duales de factores de crecimiento dentro de micropartículas de ácido poliláctico-co-glicólico (PLGA) de liberación secuencial a los hidrogeles para imitar, en parte, los eventos de señalización en la regeneración ósea: VEGF, TGF-β3, PTHrP (liberación rápida), o BMP-2, vitamina D3 (liberación lenta). La mecanotransducción se inició utilizando campos magnéticos para activar de forma remota nanopartículas superparamagnéticas (MNP) dirigidas a los canales iónicos TREK1. Los hidrogeles híbridos se implantaron subcutáneamente en ratones durante 28 días, y se evaluaron para la formación ósea utilizando micro-CT e histología. Los hidrogeles de control que carecían de HBMSCs, factores de crecimiento o MNP se mineralizaron, y ni los factores de crecimiento, ni las HBMSCs, ni la mecanotransducción aumentaron la formación ósea. Sin embargo, las diferencias estructurales en el hueso recién formado fueron influenciadas por los factores de crecimiento. La liberación lenta de BMP-2 indujo trabéculas óseas gruesas, y PTHrP o VitD3 aumentaron la formación ósea. Sin embargo, la liberación rápida de TGF-β3 y VEGF resultó en trabéculas delgadas. La mecanotransducción revirtió el adelgazamiento trabecular y aumentó la deposición de colágeno con PTHrP y VitD3. Nuestros hallazgos demuestran el potencial de los constructos de hidrogel híbrido ALG/ECM-células-factores de crecimiento para reparar el hueso en combinación con mecanotransducción para ajustar finamente la estructura ósea. Este enfoque puede formar una estrategia reparativa mínimamente invasiva para aplicaciones de ingeniería de tejidos óseos.
3729048
{3729048:TFWE8CX5}
1
nature
50
default
1706
http://www.biomateriales.ictp.csic.es/wp-content/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22TFWE8CX5%22%2C%22library%22%3A%7B%22id%22%3A3729048%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Gothard%20et%20al.%22%2C%22parsedDate%22%3A%222024-12-01%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EGothard%2C%20D.%20%3Ci%3Eet%20al.%3C%5C%2Fi%3E%20%3Ci%3EIn%20vivo%3C%5C%2Fi%3E%20analysis%20of%20hybrid%20hydrogels%20containing%20dual%20growth%20factor%20combinations%2C%20and%20skeletal%20stem%20cells%20under%20mechanical%20stimulation%20for%20bone%20repair.%20%3Ci%3EMechanobiology%20in%20Medicine%3C%5C%2Fi%3E%20%3Cb%3E2%3C%5C%2Fb%3E%2C%20100096%20%282024%29.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22%3Ci%3EIn%20vivo%3C%5C%2Fi%3E%20analysis%20of%20hybrid%20hydrogels%20containing%20dual%20growth%20factor%20combinations%2C%20and%20skeletal%20stem%20cells%20under%20mechanical%20stimulation%20for%20bone%20repair%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22David%22%2C%22lastName%22%3A%22Gothard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michael%22%2C%22lastName%22%3A%22Rotherham%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emma%20L.%22%2C%22lastName%22%3A%22Smith%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Janos%20M.%22%2C%22lastName%22%3A%22Kanczler%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22James%22%2C%22lastName%22%3A%22Henstock%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julia%20A.%22%2C%22lastName%22%3A%22Wells%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Carol%20A.%22%2C%22lastName%22%3A%22Roberts%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Omar%22%2C%22lastName%22%3A%22Qutachi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Heather%22%2C%22lastName%22%3A%22Peto%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hassan%22%2C%22lastName%22%3A%22Rashidi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Luis%22%2C%22lastName%22%3A%22Rojo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lisa%20J.%22%2C%22lastName%22%3A%22White%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Molly%20M.%22%2C%22lastName%22%3A%22Stevens%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alicia%20J.%22%2C%22lastName%22%3A%22El%20Haj%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Felicity%20R.%20A.%20J.%22%2C%22lastName%22%3A%22Rose%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Richard%20O.%20C.%22%2C%22lastName%22%3A%22Oreffo%22%7D%5D%2C%22abstractNote%22%3A%22Bone%20tissue%20engineering%20requires%20a%20combination%20of%20materials%2C%20cells%2C%20growth%20factors%20and%20mechanical%20cues%20to%20recapitulate%20bone%20formation.%20In%20this%20study%20we%20evaluated%20hybrid%20hydrogels%20for%20minimally%20invasive%20bone%20formation%20by%20combining%20biomaterials%20with%20skeletal%20stem%20cells%20and%20staged%20release%20of%20growth%20factors%20together%20with%20mechanotransduction.%20Hybrid%20hydrogels%20consisting%20of%20alginate%20and%20decellularized%2C%20demineralised%20bone%20extracellular%20matrix%20%28ALG%5C%2FECM%29%20were%20seeded%20with%20Stro-1%2B%20human%20bone%20marrow%20stromal%20cells%20%28HBMSCs%29.%20Dual%20combinations%20of%20growth%20factors%20within%20staged-release%20polylactic-co-glycolic%20acid%20%28PLGA%29%20microparticles%20were%20added%20to%20hydrogels%20to%20mimic%2C%20in%20part%2C%20the%20signalling%20events%20in%20bone%20regeneration%3A%20VEGF%2C%20TGF-%5Cu03b23%2C%20PTHrP%20%28fast%20release%29%2C%20or%20BMP-2%2C%20vitamin%20D3%20%28slow%20release%29.%20Mechanotransduction%20was%20initiated%20using%20magnetic%20fields%20to%20remotely%20actuate%20superparamagnetic%20nanoparticles%20%28MNP%29%20targeted%20to%20TREK1%20ion%20channels.%20Hybrid%20hydrogels%20were%20implanted%20subcutaneously%20within%20mice%20for%2028%20days%2C%20and%20evaluated%20for%20bone%20formation%20using%20micro-CT%20and%20histology.%20Control%20hydrogels%20lacking%20HBMSCs%2C%20growth%20factors%2C%20or%20MNP%20became%20mineralised%2C%20and%20neither%20growth%20factors%2C%20HBMSCs%2C%20nor%20mechanotransduction%20increased%20bone%20formation.%20However%2C%20structural%20differences%20in%20the%20newly-formed%20bone%20were%20influenced%20by%20growth%20factors.%20Slow%20release%20of%20BMP-2%20induced%20thick%20bone%20trabeculae%20and%20PTHrP%20or%20VitD3%20increased%20bone%20formation.%20However%2C%20fast-release%20of%20TGF-%5Cu03b23%20and%20VEGF%20resulted%20in%20thin%20trabeculae.%20Mechanotransduction%20reversed%20the%20trabecular%20thinning%20and%20increased%20collagen%20deposition%20with%20PTHrP%20and%20VitD3.%20Our%20findings%20demonstrate%20the%20potential%20of%20hybrid%20ALG%5C%2FECM%20hydrogel%5Cu2013cell%5Cu2013growth%20factor%20constructs%20to%20repair%20bone%20in%20combination%20with%20mechanotransduction%20for%20fine-tuning%20bone%20structure.%20This%20approach%20may%20form%20a%20minimally%20invasive%20reparative%20strategy%20for%20bone%20tissue%20engineering%20applications.%22%2C%22date%22%3A%222024-12-01%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.mbm.2024.100096%22%2C%22ISSN%22%3A%222949-9070%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fpii%5C%2FS2949907024000597%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222024-09-30T10%3A32%3A59Z%22%7D%7D%5D%7D
1.
Gothard, D. et al. In vivo analysis of hybrid hydrogels containing dual growth factor combinations, and skeletal stem cells under mechanical stimulation for bone repair. Mechanobiology in Medicine 2, 100096 (2024).