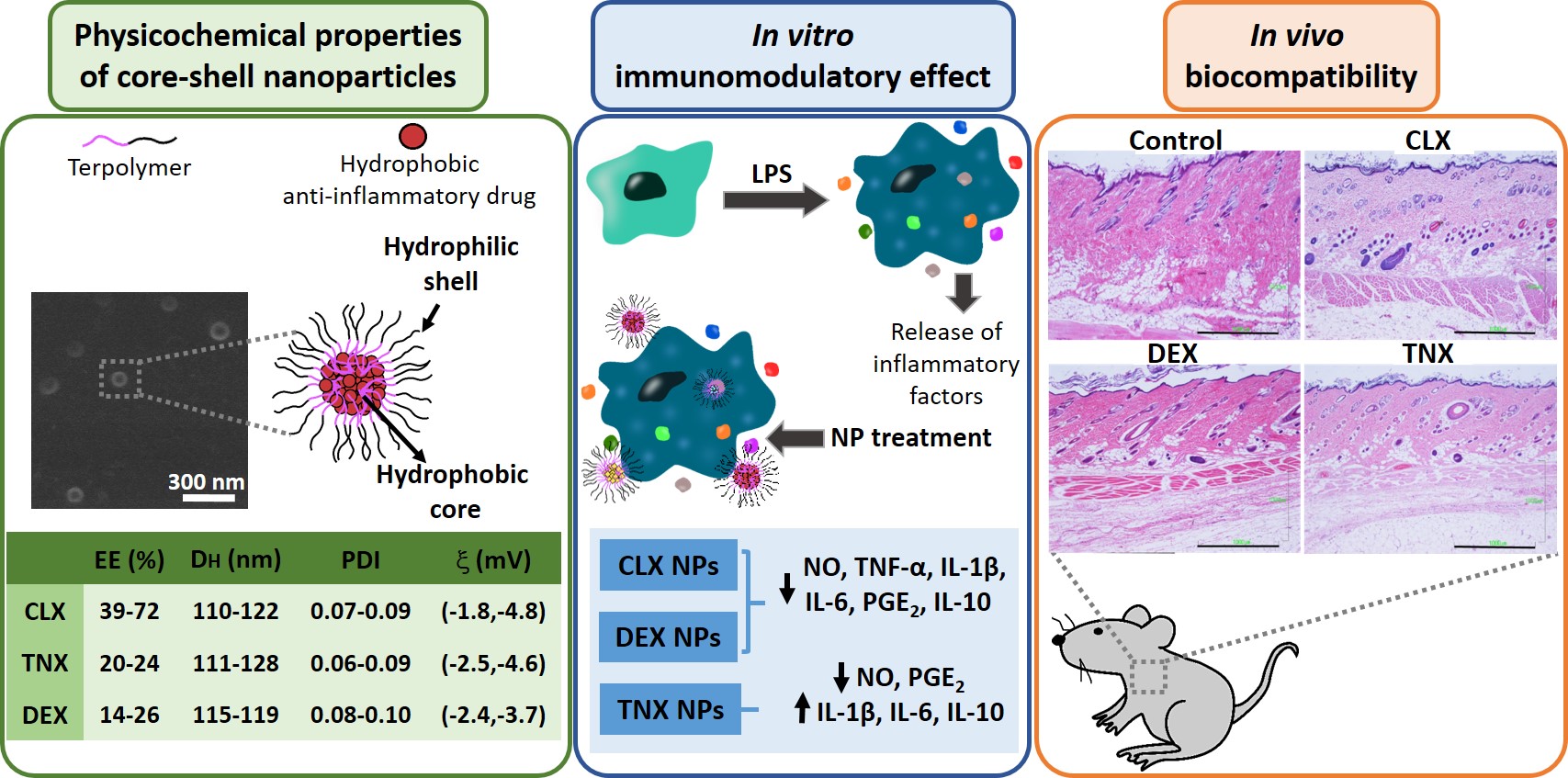
Los tratamientos de primera línea de la osteoartritis se basan en fármacos anti-inflamatorios, siendo los más usados los anti-inflamatorios no esteroideos (AINEs), los inhibidores específicos de COX-2 y los corticoides. La mayoría de ellos presenta citotoxicidad y una baja biodisponibilidad en condiciones fisiológicas, haciendo necesaria la administración de altas concentraciones que provocan diferentes efectos secundarios. El objetivo de este trabajo ha sido encapsular tres anti-inflamatorios hidrofóbicos de diferentes naturalezas (celecoxib, tenoxicam y dexametasona) en nanopartículas poliméricas con potencial aplicación en osteoartritis. Las nanopartículas presentan tamaños entre 110 y 130 nm, con cargas neutras (entre -1 y -5 mV). La eficiencia de encapsulación demostró ser altamente dependiente del fármaco encapsulado y de su solubilidad en agua, obteniendo los mayores valores para el celecoxib (39-72%) seguido del tenoxicam (20-24%) y de la dexametasona (14-26%). La nanoencapsulación redujo la citotoxicidad del celecoxib y la dexametasona en condrocitos articulares humanos y macrófagos murinos RAW264.7. Además, los tres sistemas cargados mostraron ser no citotóxicos en un amplio rango de concentraciones. Las nanopartículas cargadas con celecoxib y dexametasona redujeron la liberación de diferentes factores inflamatorios (NO, TNF-α, IL-1β, IL-6, PGE2 y IL-10) en RAW264.7 estimulada con LPS. Las nanopartículas cargadas con tenoxicam redujeron la producción de NO y PGE2 , aunque se observó una sobreexpresión de las IL-1β, IL-6 e IL-10. Finalmente, todas las nanopartículas demostraron ser biocompatibles en un modelo de rata mediante inyección subcutánea. Estos descubrimientos sugieren que estas nanopartículas cargadas pueden ser candidatas para el tratamiento de procesos inflamatorios relacionados con la osteoartritis debido a su demostrada actividad in vitro como reguladores de la producción de mediadores inflamatorios.
3729048
{3729048:EW2Z4W7W}
1
nature
50
default
1
1
title
887
http://www.biomateriales.ictp.csic.es/wp-content/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22EW2Z4W7W%22%2C%22library%22%3A%7B%22id%22%3A3729048%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Pontes-Quero%20et%20al.%22%2C%22parsedDate%22%3A%222021-02%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20%5C%22%3E%5Cn%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E1.%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EPontes-Quero%2C%20G.%20M.%2C%20Benito-Garz%5Cu00f3n%2C%20L.%2C%20P%5Cu00e9rez%20Cano%2C%20J.%2C%20Aguilar%2C%20M.%20R.%20%26%20V%5Cu00e1zquez-Lasa%2C%20B.%20%3Ca%20class%3D%27zp-ItemURL%27%20target%3D%27_blank%27%20href%3D%27https%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F1999-4923%5C%2F13%5C%2F2%5C%2F290%27%3EModulation%20of%20Inflammatory%20Mediators%20by%20Polymeric%20Nanoparticles%20Loaded%20with%20Anti-Inflammatory%20Drugs%3C%5C%2Fa%3E.%20%3Ci%3EPharmaceutics%3C%5C%2Fi%3E%20%3Cb%3E13%3C%5C%2Fb%3E%2C%20290%20%282021%29.%20%3Ca%20title%3D%27Cite%20in%20RIS%20Format%27%20class%3D%27zp-CiteRIS%27%20href%3D%27http%3A%5C%2F%5C%2Fwww.biomateriales.ictp.csic.es%5C%2Fwp-content%5C%2Fplugins%5C%2Fzotpress%5C%2Flib%5C%2Frequest%5C%2Frequest.cite.php%3Fapi_user_id%3D3729048%26amp%3Bitem_key%3DEW2Z4W7W%27%3ECite%3C%5C%2Fa%3E%20%3C%5C%2Fdiv%3E%5Cn%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Modulation%20of%20Inflammatory%20Mediators%20by%20Polymeric%20Nanoparticles%20Loaded%20with%20Anti-Inflammatory%20Drugs%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gloria%20Mar%5Cu00eda%22%2C%22lastName%22%3A%22Pontes-Quero%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lorena%22%2C%22lastName%22%3A%22Benito-Garz%5Cu00f3n%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Juan%22%2C%22lastName%22%3A%22P%5Cu00e9rez%20Cano%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mar%5Cu00eda%20Rosa%22%2C%22lastName%22%3A%22Aguilar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Blanca%22%2C%22lastName%22%3A%22V%5Cu00e1zquez-Lasa%22%7D%5D%2C%22abstractNote%22%3A%22The%20first-line%20treatment%20of%20osteoarthritis%20is%20based%20on%20anti-inflammatory%20drugs%2C%20the%20most%20currently%20used%20being%20nonsteroidal%20anti-inflammatory%20drugs%2C%20selective%20cyclooxygenase%202%20%28COX-2%29%20inhibitors%20and%20corticoids.%20Most%20of%20them%20present%20cytotoxicity%20and%20low%20bioavailability%20in%20physiological%20conditions%2C%20making%20necessary%20the%20administration%20of%20high%20drug%20concentrations%20causing%20several%20side%20effects.%20The%20goal%20of%20this%20work%20was%20to%20encapsulate%20three%20hydrophobic%20anti-inflammatory%20drugs%20of%20different%20natures%20%28celecoxib%2C%20tenoxicam%20and%20dexamethasone%29%20into%20core-shell%20terpolymer%20nanoparticles%20with%20potential%20applications%20in%20osteoarthritis.%20Nanoparticles%20presented%20hydrodynamic%20diameters%20between%20110%20and%20130%20nm%20and%20almost%20neutral%20surface%20charges%20%28between%20%5Cu22121%20and%20%5Cu22125%20mV%29.%20Encapsulation%20efficiencies%20were%20highly%20dependent%20on%20the%20loaded%20drug%20and%20its%20water%20solubility%2C%20having%20higher%20values%20for%20celecoxib%20%2839%5Cu201372%25%29%20followed%20by%20tenoxicam%20%2820%5Cu201324%25%29%20and%20dexamethasone%20%2814%5Cu201326%25%29.%20Nanoencapsulation%20reduced%20celecoxib%20and%20dexamethasone%20cytotoxicity%20in%20human%20articular%20chondrocytes%20and%20murine%20RAW264.7%20macrophages.%20Moreover%2C%20the%20three%20loaded%20systems%20did%20not%20show%20cytotoxic%20effects%20in%20a%20wide%20range%20of%20concentrations.%20Celecoxib%20and%20dexamethasone-loaded%20nanoparticles%20reduced%20the%20release%20of%20different%20inflammatory%20mediators%20%28NO%2C%20TNF-%5Cu03b1%2C%20IL-1%5Cu03b2%2C%20IL-6%2C%20PGE2%20and%20IL-10%29%20by%20lipopolysaccharide%20%28LPS%29-stimulated%20RAW264.7.%20Tenoxicam-loaded%20nanoparticles%20reduced%20NO%20and%20PGE2%20production%2C%20although%20an%20overexpression%20of%20IL-1%5Cu03b2%2C%20IL-6%20and%20IL-10%20was%20observed.%20Finally%2C%20all%20nanoparticles%20proved%20to%20be%20biocompatible%20in%20a%20subcutaneous%20injection%20model%20in%20rats.%20These%20findings%20suggest%20that%20these%20loaded%20nanoparticles%20could%20be%20suitable%20candidates%20for%20the%20treatment%20of%20inflammatory%20processes%20associated%20with%20osteoarthritis%20due%20to%20their%20demonstrated%20in%20vitro%20activity%20as%20regulators%20of%20inflammatory%20mediator%20production.%22%2C%22date%22%3A%222021%5C%2F2%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.3390%5C%2Fpharmaceutics13020290%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F1999-4923%5C%2F13%5C%2F2%5C%2F290%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222021-02-23T15%3A23%3A50Z%22%7D%7D%5D%7D