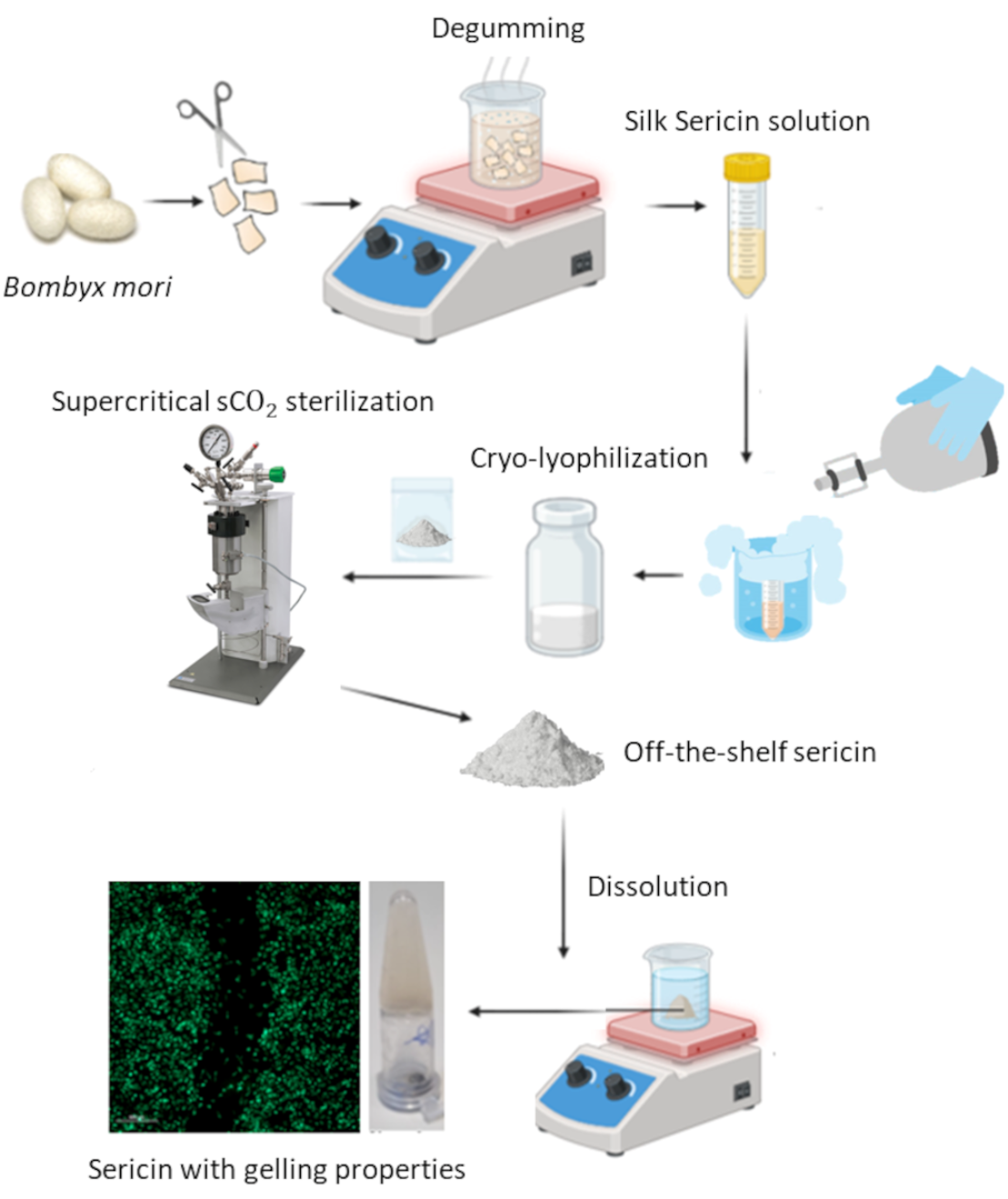
Silk sericin (SS), a by-product of the textile industry, has gained significant attention for its biomedical potential due to its biocompatibility and regenerative potential. However, the literature lacks information on SS processing methods and the resulting physicochemical properties. This study represents the first step in protocol optimization and standardization. In the present work, different processing techniques were studied and compared on SS extracted from boiling water: evaporation, rotary evaporation, lyophilization, and dialysis, which presented a recovery yield of approximately 27–32%. The goal was to find the most promising process to concentrate extracted SS solutions, and to ensure that the SS structure was highly preserved. As a result, a new cryo-lyophilization methodology was proposed. The proposed method allows for the preservation of the amorphous structure, which offers significant advantages including complete dissolution in water and PBS, an increase in storage stability, and the possibility of scaling-up, making it highly suitable for industrial and biomedical applications. The second part of the work focused on addressing another challenge in SS processing: efficient and non-destructive sterilization. Supercritical CO2 (scCO2) has been gaining momentum in the last years for sterilizing sensitive biopolymers and biological materials due to its non-toxicity and mild processing conditions. Thus, scCO2 technology was validated as a mild technique for the terminal sterilization of SS. In this way, it was possible to engineer a sequential cryo-lyophilization/scCO2 sterilization process which was able to preserve the original properties of this natural silk protein. Overall, we have valorized SS into a sterile, off-the-shelf, bioactive, and water-soluble material, with the potential to be used in the biomedical, pharmaceutical, or cosmetic industries.
Nanocarrier of α-Tocopheryl Succinate Based on a Copolymer Derivative of (4,7-dichloroquinolin-2-yl)methanol and Its Cytotoxicity against a Breast Cancer Cell Line
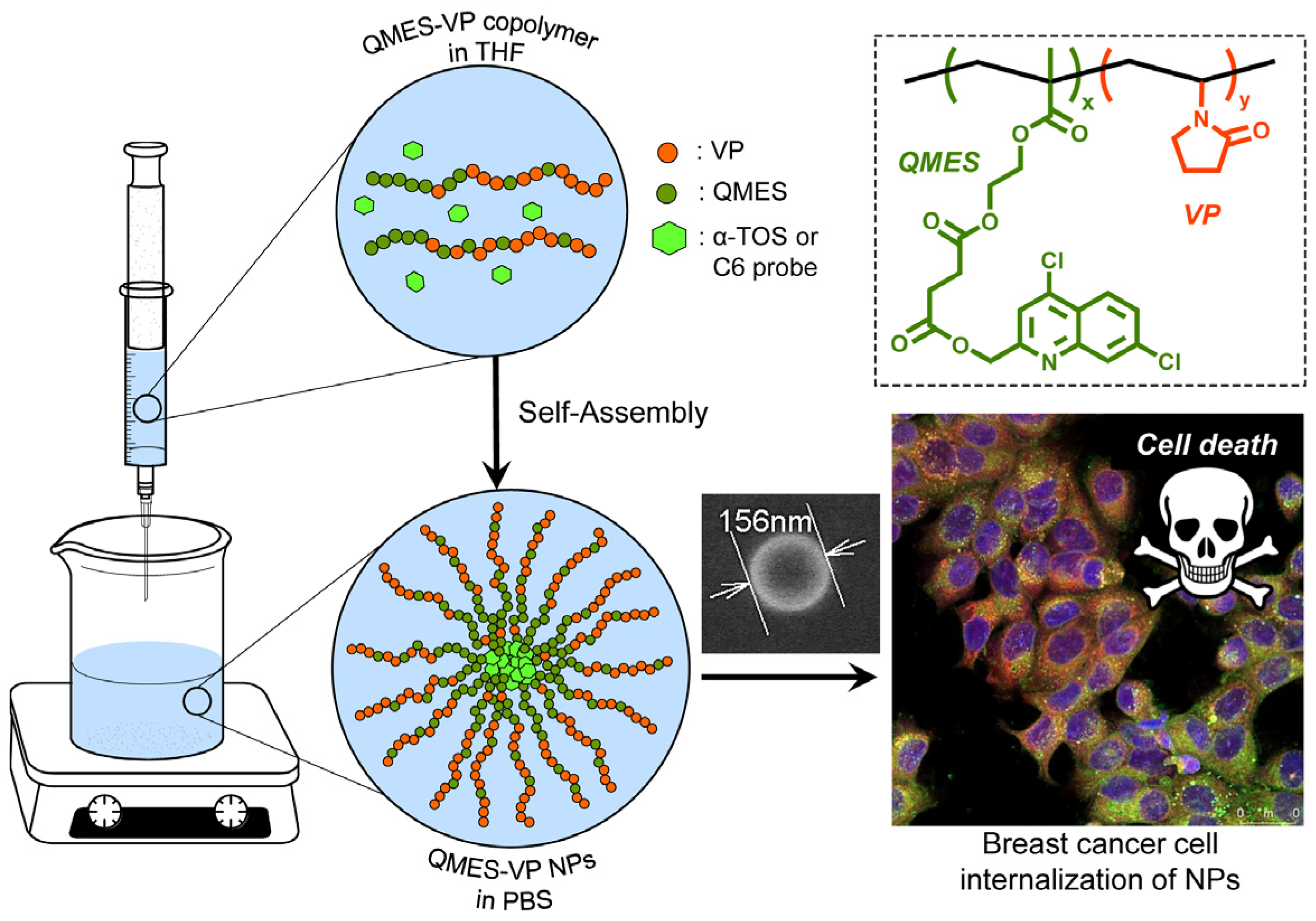
In order to improve the water solubility and, therefore, bioavailability and therapeutic activity of anticancer hydrophobic drug α-tocopherol succinate (α-TOS), in this work, copolymers were synthesized via free radicals from QMES (1-[4,7-dichloroquinolin-2-ylmethyl]-4-methacryloyloxyethyl succinate) and VP (N-vinyl-2-pirrolidone) using different molar ratios, and were used to nanoencapsulate and deliver α-TOS into cancer cells MCF-7. QMES monomer was chosen because the QMES pendant group in the polymer tends to hydrolyze to form free 4,7-dichloro-2-quinolinemethanol (QOH), which also, like α-TOS, exhibit anti-proliferative effects on cancerous cells. From the QMES-VP 30:70 (QMES-30) and 40:60 (QMES-40) copolymers obtained, it was possible to prepare aqueous suspensions of empty nanoparticles (NPs) loaded with α-TOS by nanoprecipitation. The diameter and encapsulation efficiency (%EE) of the QMES-30 NPs loaded with α-TOS were 128.6 nm and 52%; while for the QMES-40 NPs loaded with α-TOS, they were 148.8 nm and 65%. The results of the AlamarBlue assay at 72 h of treatment show that empty QMES-30 NPs (without α-TOS) produced a marked cytotoxic effect on MCF-7 breast cancer cells, corresponding to an IC50 value of 0.043 mg mL−1, and importantly, they did not exhibit cytotoxicity against healthy HUVEC cells. Furthermore, NP-QMES-40 loaded with α-TOS were cytotoxic with an IC50 value of 0.076 mg mL−1, demonstrating a progressive release of α-TOS; however, the latter nanoparticles were also cytotoxic to healthy cells in the range of the assayed concentrations. These results contribute to the search for a new polymeric nanocarrier of QOH, α-TOS or other hydrophobic drugs for the treatment of cancer or others diseases treatable with these drugs.
Nanoparticles of 4,7-dichloro-2-quinolinemethylacrylate-based copolymers and their potential cytotoxic activity on human breast carcinoma cells
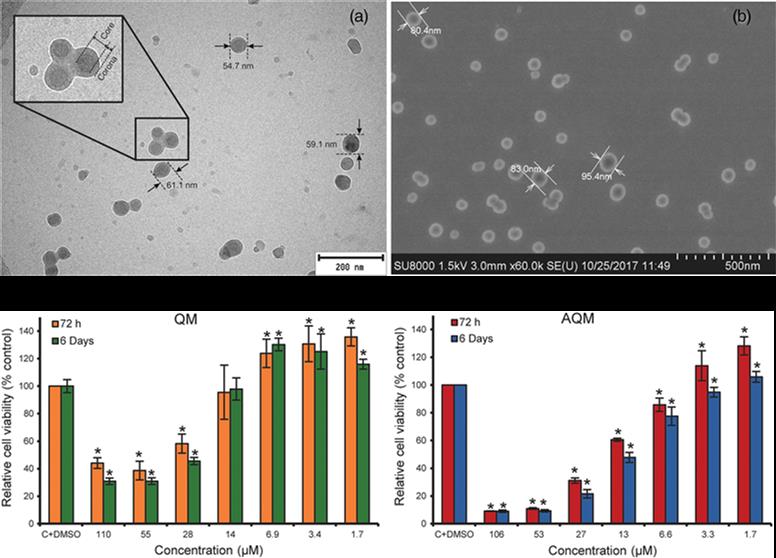
In this article, an improved synthesis strategy of the potent anticancer compound 4,7-dichloro-2-quinolinemethanol (QM) and its acrylate ester 4,7-dichloro-2-quinolinemethylacrylate (AQM) are described. AQM is copolymerized using free-radical polymerization with N-vinyl-2-pyrrolidone (VP) and the copolymers obtained from different molar ratios of monomers are subjected to nanoprecipitation to produce suspensions of nanoparticles (NPs) in phosphate buffered saline (PBS). The smallest and stable NPs are
prepared with the AQM-VP copolymers 45:55 and 40:60 (118.9 and 128.7 nm in diameter, respectively) at 1 mg mL−1, and along with AQM and QM, are evaluated for their cytotoxic activity on MDA-MB-453 breast carcinoma cells using MTT bioassay. AQM and QM are highly cytotoxic (IC50: 19 and 41 μM, respectively); however, the NPs are not cytotoxic in the range of the assayed concentrations. These results contribute to the search for new polymeric NPs with potential application as QM delivery systems for the treatment of cancer or other diseases treatable with QM.
1.Valle, H. et al. Nanoparticles of 4,7-dichloro-2-quinolinemethylacrylate-based copolymers and their potential cytotoxic activity on human breast carcinoma cells. Journal of Applied Polymer Science 136, 47545 (2019). Cite